
1
This content does not meet HHS and OS accessibility standards. For immediate
assistance, please contact NHSN@cdc.gov
Guidance for Hospitals and Acute Care Facilities Reporting of
Respiratory Pathogen, Bed Capacity, and Supply Data to CDC’s
National Healthcare Safety Network (NHSN)
Updated: November 17, 2023
Implementation Date: November 26, 2023
[CHANGE] Note: For ease of navigation, all changes as of this November 17, 2023 guidance have been
highlighted with [CHANGE]. This guidance update reflects the addition of new data elements available
for optional reporting to CDC's National Healthcare Safety Network (NHSN) of information related to
laboratory-confirmed influenza and laboratory-confirmed respiratory syncytial virus (RSV) infection.
There are no additions of required data elements or changes to existing data elements as a result of this
guidance update. Information on reporting to NHSN can be found here:
https://www.cdc.gov/nhsn/covid19/hospital-reporting.html.
Since March 29, 2020, the U.S. government has been collecting data from hospitals and states to
understand health care system stress, capacity, capabilities, and the number of patients hospitalized due to
COVID-19. As COVID-19 continues to evolve, Federal needs for data are also evolving. In an effort to
reduce burden while maximizing efficiency, the Federal government continues to evaluate data needs. All
data collected are driven by two core principles: 1) the data must drive action and/or 2) the data must
serve as a surveillance indicator for U.S. health care system stress, capacity, capability, and/or patient
safety. Significant consideration is also given to align with state, tribal, local, and territorial (STLT) needs
wherever possible and to minimize system changes and/or disruptions.
All hospitals have been required to report COVID-19 data daily to the Federal Government under the
CMS’ Conditions of Participation since September 2, 2020. Under the initial CMS Interim Final Rules
from 2020, the required reporting was scheduled to end at the conclusion of the COVID-19 Public Health
Emergency (PHE), which expired on May 11, 2023. On August 10, 2022, CMS finalized the annual IPPS
rule to amend the required reporting, which had several impacts for COVID-19 required reporting from
all hospitals:
• It extended reporting from the end of the current PHE through April 30, 2024, unless the
Secretary of the Department of Health and Human Services establishes an earlier end-date.
• It indicated that the number of required data elements would be fewer after the end of the PHE.
• It indicated that reporting submission would not be daily after the end of the PHE.
[CHANGE] The COVID-19 pandemic has underscored the public health threat of respiratory pathogens
and highlighted the need for comprehensive, real-time data for prevention and response purposes. In
addition to COVID-19, seasonal influenza and RSV can result in substantial burden on hospitals. For
those reason, following the expiration of the PHE in May 2023, and prior to the fall 2023 respiratory virus
season, optional collection of additional influenza data elements and new data elements capturing
information on respiratory syncytial virus (RSV) are now available for reporting as part of the COVID-19
hospital data collection through the Centers for Disease Control and Prevention (CDC)’s National
2
Healthcare Safety Network (NHSN). The addition of flu and RSV optional data fields can be used to
improve situational awareness of severe respiratory illness and assess potential impact of flu, COVID-19,
and RSV co-circulation, allow for hospitalization forecasting, resource allocation, and help inform
guidance and recommendations for public health professionals, clinicians, and the general public.
Understanding influenza and RSV hospitalizations and admissions can also help to understand potential
strains on the PPE supply chain.
This November 17, 2023 guidance update reflects additions to the COVID-19 hospital data reporting, to
include optional data fields for reporting influenza infection and RSV infection for adult and pediatric:
• New hospital admissions
• Hospitalized patients
• Hospitalized ICU patients
Reporting for the new influenza and RSV fields is optional and voluntary. The addition of the influenza
and RSV fields does not impact the FY 2023 Hospital Inpatient Prospective Payment System (IPPS) and
Long-Term Care Hospital Prospective Payment System (LTCH PPS) Final Rule (CMS-1771-F) for
hospital, critical access hospital (CAH), psychiatric facility, and rehabilitation facility infection
prevention and control conditions of participation (CoP) requirements for hospital COVID-19 data
reporting. Current processes for reporting hospital COVID-19 data to NHSN can continue exactly as is.
The following details the data elements, cadence, and how the data are being used in the federal response.
Appendix A includes a change log for comparison to previous hospital reporting guidance.
Who is responsible for reporting, and when is reporting required?
As of December 15, 2022, hospitals are responsible for reporting the information directly to the Federal
government using the Centers for Disease Control and Prevention (CDC)’s National Healthcare Safety
Network (NHSN). Facilities should report at the individual hospital level, even if hospitals share a
Centers for Medicare & Medicaid Services (CMS) Certification Number (CCN).
We recognize that some health care systems choose to report for all facilities in their network from a
central corporate location.
We also recognize that many states currently collect this information from the hospitals in their
jurisdiction. Therefore, hospitals may be relieved from reporting directly to the Federal government if
they receive a written release from the state indicating that the state is certified and will collect the data
from the hospitals and take over the hospital’s Federal reporting responsibilities. STLT partners may have
unique reporting requirements either related to or independent of the Federal reporting requirements.
Facilities are encouraged to work with their relevant STLT partners to ensure complete reporting.
To be considered “certified”, states must first receive written certification from their Administration for
Strategic Preparedness and Response (ASPR) Regional Administrator affirming that the state has an
established, functioning data reporting stream to the federal government that is delivering all information
shown in the table below at the appropriate daily frequency. States that take over reporting must provide
these data, regardless of whether they are seeking immediate federal assistance. States that are certified
are listed on healthdata.gov.
3
Cadence and Facility Type
As of the June 11, 2023 guidance, all hospitals (except psychiatric and rehabilitation hospitals) are
required to report daily data values weekly to NHSN. The weekly data submission should be submitted by
Tuesday, 11:59pm local time, and include daily data for each day in the previous week, defined as the
previous Sunday through Saturday. Example calendar is displayed below to demonstrate the weekly
reporting cadence change.
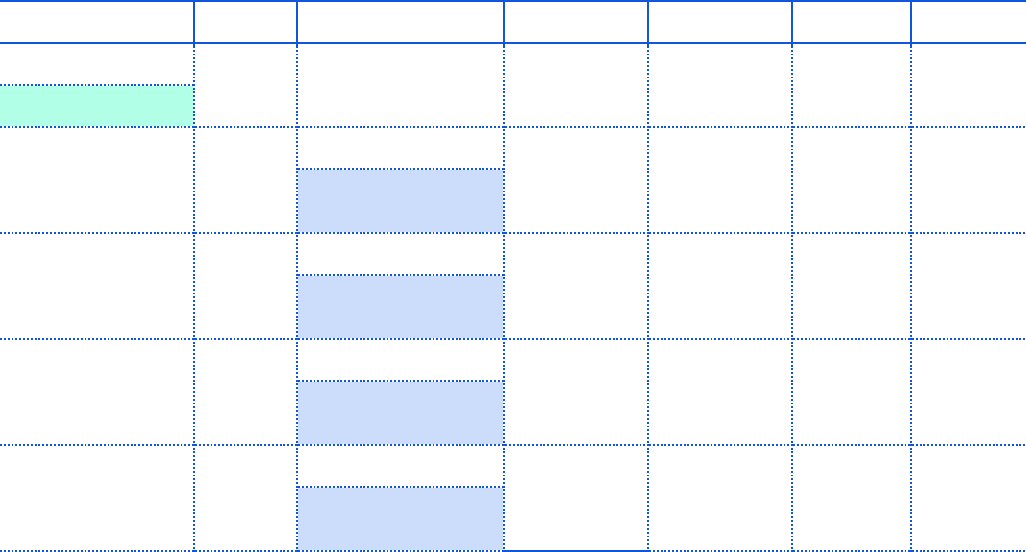
4
Example calendar view for reporting cadence:
Sunday
Monday
Tuesday
Wednesday
Thursday
Friday
Saturday
June 11
June 12
June 13
June 14
June 15
June 16
June 17
Implementation date
June 18
June 19
June 20
June 21
June 22
June 23
June 24
Weekly submission
deadline (June 11-17)
June 25
June 26
June 27
June 28
June 29
June 30
July 1
Weekly submission
deadline (June 18-24)
July 2
July 3
July 4**
July 5
July 6
July 7
July 8
Weekly submission
deadline (June 25-July 1)
July 9
July 10
July 11
July 12
July 13
July 14
July 15
Weekly submission
deadline (July –July 8)
**For Tuesday deadlines falling on federal holidays, the reporting deadline will shift to Wednesday of the
same week.
Data should NOT be aggregated to weekly values; values for each day in the reporting period should be
reported separately.
All hospitals are asked to follow the direction of their state and jurisdiction to ensure reporting
meets STLT needs.
It is critical that the data are reported by Tuesday for the previous week (Sunday-Saturday) to count
towards compliance. For daily required fields, data must be submitted for each day in the reporting week;
for weekly required fields, data must be submitted for Wednesday in the reporting week, in order to count
for compliance. Weekly required reporting of daily values allows for ongoing collection of reliable data
needed for understanding severity and burden of COVID-19 on healthcare facilities and state of
healthcare capacity in the United States.
Data can be submitted at any point during the enforcement period; reporters do not need to wait until
Tuesday to submit their data.
5
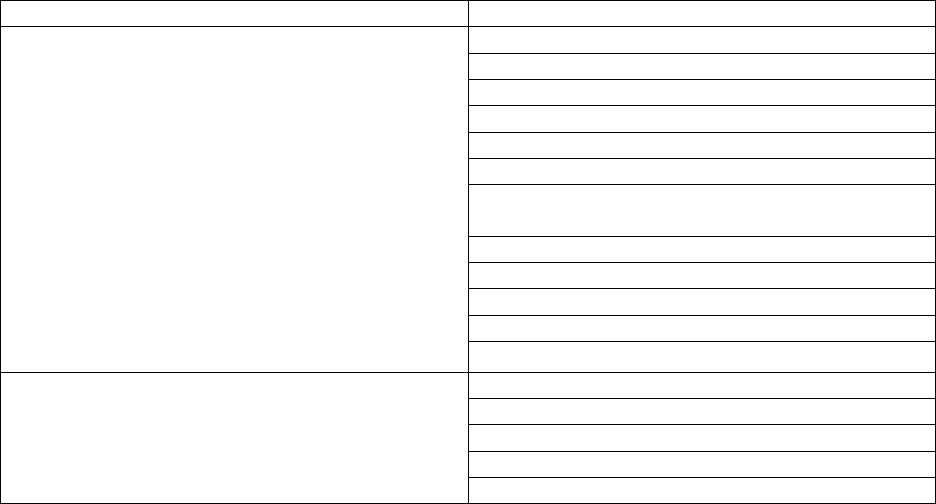
Reporting Cadence
Facility description
Weekly by Tuesday 1159pm local time; data for
each during in the previous week (defined as
previous Sunday through Saturday) should be
included for daily required fields, and for
Wednesday for weekly required fields
Short-term Acute Care Hospitals
Medicaid Only Short-term Hospitals
Long-term Care Hospitals
Critical Access Hospitals
Children’s Hospitals
Medicaid Only Children’s Hospitals
General Hospitals (including acute, trauma, and
teaching)
Women’s Hospitals
Oncology Hospitals
Military Hospitals
Indian Health Service Hospitals
Veteran’s Administration Hospitals
Per Secretary discretion, Psychiatric and
rehabilitation facility federal reporting has been
set to submitting data once annually from October
to October.
Psychiatric Hospitals
Distinct Part Psych Hospitals
Medicaid Only Psychiatric Hospitals
Rehabilitation Hospitals
Medicaid Only Rehabilitation Hospitals
*We recognize that STLT partners may have reporting requirements related to or independent of the Federal
reporting requirements. Facilities are encouraged to work with relevant STLT partners to ensure complete
reporting for all partners. All hospitals are asked to follow the direction of their state and jurisdiction to
ensure reporting meets STLT needs.
Reporting Flexibilities
We recognize that reporting requires staffing resources and have implemented the following flexibilities.
All hospitals are asked to follow the direction of their state and jurisdiction to ensure reporting
meets STLT needs.
Holidays: Pending further direction from their state or jurisdiction, hospitals are not expected to report to
the Federal government on holidays unless otherwise noted; however, hospitals are requested
to report the data elements by the next Wednesday immediately following the holiday,
backdated to the appropriate date. All hospitals are asked to follow the direction of their state
and jurisdiction to ensure reporting meets STLT needs.
Weekends: Where possible and pending further direction from their state or jurisdiction, hospitals are not
expected to report on weekends; however, hospitals are requested to report the data elements
by the next Tuesday submission deadline immediately following the weekend, backdated to
the appropriate date. All hospitals are asked to follow the direction of their state and
jurisdiction to ensure reporting meets STLT needs.
Emergencies: Hospitals experiencing additional natural and/or manmade disasters such as wildfires,
hurricanes, cyber incidents, flooding, etc. can be placed in emergency suspense. Facilities
placed in emergency suspense are not required to report COVID-19 data for the duration
of the suspense. Backdated reporting is not required after the incident is resolved.
6
How to Report
Hospitals should report information to the Federal government through one of the methods below
1
.
Options are provided to best meet facility needs. Facilities should report at the individual hospital level,
even if hospitals share a CCN. To view the most recent templates, view the templates located on the
NHSN webpage and accompanying resources: https://www.cdc.gov/nhsn/covid19/hospital-
reporting.html.https://www.cdc.gov/nhsn/covid19/hospital-reporting.html. Additional technical materials
can also be found on https://healthdata.gov/stories/s/kjst-g9cm.
As of December 15, 2022, COVID-19 hospital data are collected through CDC’s National Healthcare
Safety Network (NHSN). Jurisdictions are able to submit data on behalf of facilities within their area,
hospital systems can submit data at an enterprise level, third-party providers can submit data on behalf of
facilities and/or jurisdictions, and hospitals can report individually. Reporting capabilities for a web
interface, CSV upload, and API are available.
Method
Description
State Certification
If your state has assumed reporting responsibility, submit all data to your state
each day, and your state will submit on your behalf. Your state can provide you
with a certification if they are authorized to submit on your behalf. States are
able to submit data via any of the below mechanisms (submitting data to
NHSN, centralized reporting system, and/or health IT vendors or another third-
party).
Submit Data to
NHSN
As of December 15, 2022, COVID-19 hospital data collection was transitioned
to NHSN. Instructions and recordings for submitting COVID-19 hospital data
to NHSN are available on the NHSN website. Instructions and recordings for
submitting COVID-19 hospital data to NHSN are available on the NHSN
website.
Centralized System
Reporting to NHSN
Centralized reporting is available for entities reporting data on behalf of
multiple facilities. If you are an individual hospital, hospital organization or
state reporting many facilities, use the available template. Note: The primary
template is identical to the previous template used to submit data to
TeleTracking.
Share Information
Directly with NHSN
through your Health
IT Vendor or Other
Third-Party
Individual hospitals and/or hospital organizations may provide authorization to
a third-party vendor for Health IT, emergency management, situational
awareness, and/or other provider for sharing data directly with HHS through
NHSN on behalf of the facility.
Note: Specific information is requested through different systems and mechanisms, such as therapeutics
data through HPOP and testing data through public health mechanisms.
Troubleshooting & Operational Status Changes
Hospitals that encounter reporting challenges, have name changes and/or changes in operational status s
should contact the NHSN helpdesk (nhsn@cdc.gov with subject line COVID19 Hospital) for assistance.
Newly established hospitals and/or hospitals with new ownership are granted a 30-day reporting
exemption to establish reporting mechanisms and protocols.
1
Note: Posting information publicly to hospital and/or hospital organization website using common data standards
was previously provided as an option for submitting data. This option has been removed as it was not utilized.

7
Data Elements
The following data elements help the Federal government understand health care system stress, capacity,
capabilities, and the number of patients hospitalized due to COVID-19. Data elements may be required or
optional and may be associated with a specific cadence. The purpose of each data element and how it
informs the Federal response is in Appendix B.
Required Data Elements: These data elements are requested from facilities to ensure a complete data
submission. Any associated Federal compliance is evaluated on required data elements only. Some data
elements are requested at each reporting interval (i.e., daily), while others are requested weekly.
Optional Data Elements: Hospital reporting on these fields is determined at a jurisdiction and/or facility
level. Hospitals are asked to follow the direction of their STLT government on reporting these fields;
otherwise, reporting is at the discretion of the facility for the purposes of federal reporting.
2
These data
elements are helpful to the federal response and may be used for additional analyses and planning
purposes. Note: Hospitals can continue reporting data on these fields- the fields are not being removed
from templates.
Federally Inactive Data Elements: These data elements have been made inactive for the federal data
collection and are no longer required at the federal level. Hospitals are asked to follow the direction of
their STLT government on reporting these fields, as some jurisdictions may choose to keep certain data
elements as part of the collection based on their needs. Note: Hospitals can continue reporting data on
these fields- the fields are not being removed from templates.
Daily Data Elements: Hospitals are requested to provide information on these data elements by Tuesday
for each day during the previous reporting week (Sunday through Saturday).
Weekly Data Elements: Hospitals are requested to provide information on these data elements once per
week for Wednesdays. Weekly data elements must be provided on Wednesday to count towards
compliance requirements. Wednesday data can be included in the weekly submission. The data for the
previous Wednesday would be included in the submission for that week.
Example:
Data for Sunday, June 11 – Saturday, June 17 would need to be submitted by end of day Tuesday, June
20. The required data for Wednesday, June 14 would be included in the submission due by end of day
Tuesday, June 20.
The data elements are listed in the table below by data field ID number and grouped by category:
Metadata, Capacity, Supply, Influenza, Therapeutic, Therapeutic Placeholder, and Healthcare Worker
Vaccination. The data element description, whether the field is required or optional, and the requested
cadence are indicated. A list of data elements grouped by cadence and whether they are required or
optional is available in Appendix C.
Changes to data elements are also indicated throughout the document where appropriate, in addition to the
change log in Appendix A.
2
We recognize that STLT partners may have reporting requirements related to or independent of the Federal
reporting requirements. Facilities are encouraged to work with relevant STLT partners to ensure complete reporting
for all partners.

8
• [CHANGE] Data elements that are new in this November 17, 2023 guidance are marked as
[NEW] and highlighted within the table.
• There are no new required data elements or existing data elements that have been made
optional or inactive for the federal data collection as of this November 17, 2023 guidance;
data elements that were previously made inactive for the federal data collection are also
highlighted in italics and in gray.
• Previous changes (dated June 11, 2023) are no longer marked as [CHANGE].
The purpose of each data element is available in Appendix B.
Additional details on the data elements are available in Appendix D. A visual representation of related
capacity and occupancy fields is available in Appendix E.
9
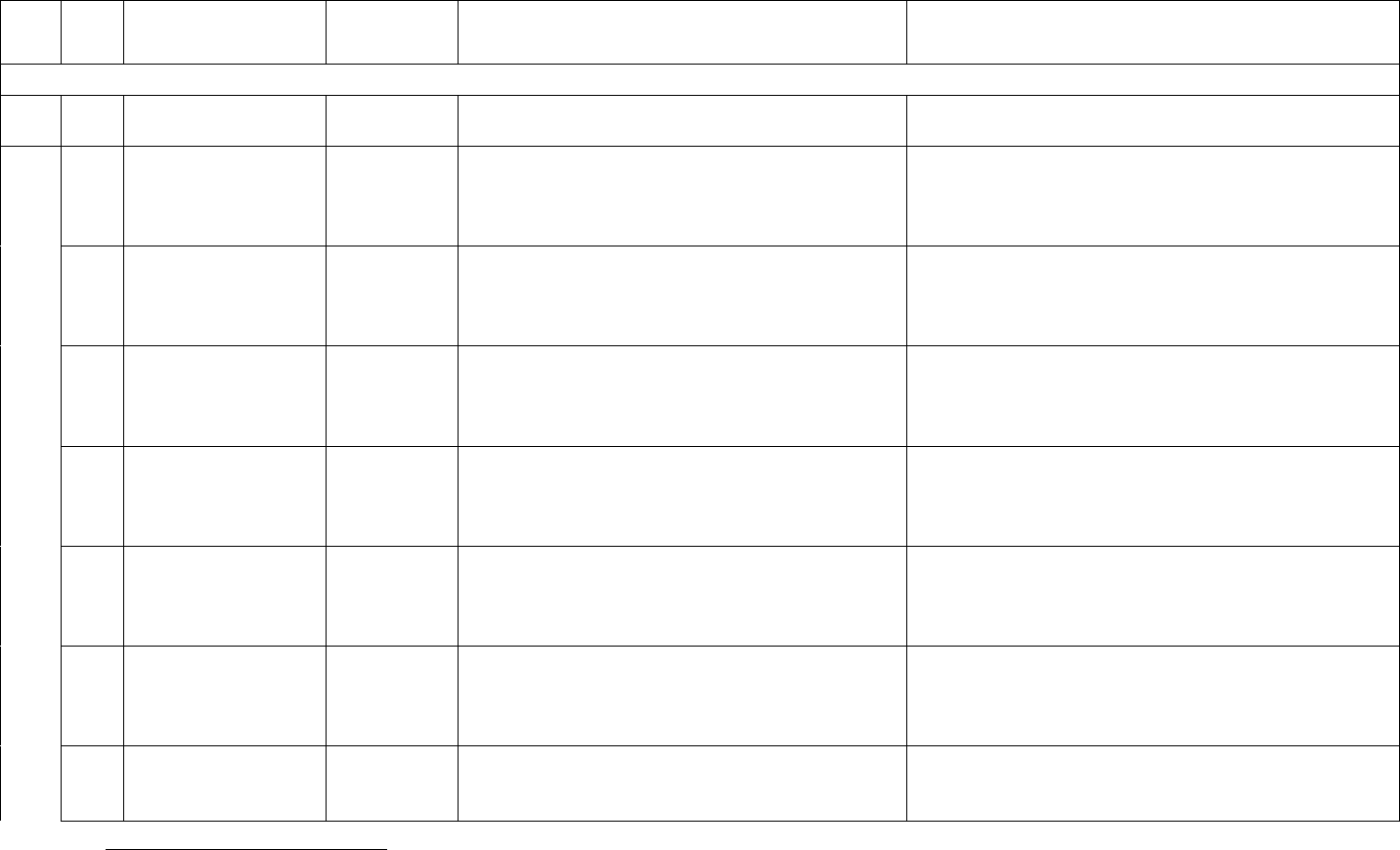
Data Element Table
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
Metadata
3
ID
Sub
ID
Required/Optional
Cadence
Information Needed
Description
1
a.
Required
Weekly, for
all days in
previous
week
Hospital Name
Name of hospital
1
b.
Required
Weekly, for
all days in
previous
week
CCN
Hospital CMS Certification Number (CCN)
1
c.
Required
Weekly, for
All days in
previous
week
NHSN Org ID
The NHSN-assigned facility ID
Note: NHSN Org ID is needed to submit data into the
NHSN system
1
d.
Required
Weekly, for
All days in
previous
week
State
State where the hospital is located
1
e.
Required
Weekly, for
All days in
previous
week
County
County where the hospital is located
1
f.
Required
Weekly, for
All days in
previous
week
ZIP
ZIP where the hospital is located
1
g.
Optional
Weekly, for
All days in
previous
TeleTracking ID
The identifier assigned by TeleTracking
3
Entities reporting on behalf of facilities are encouraged to auto-populate the relevant information on behalf of the facility.
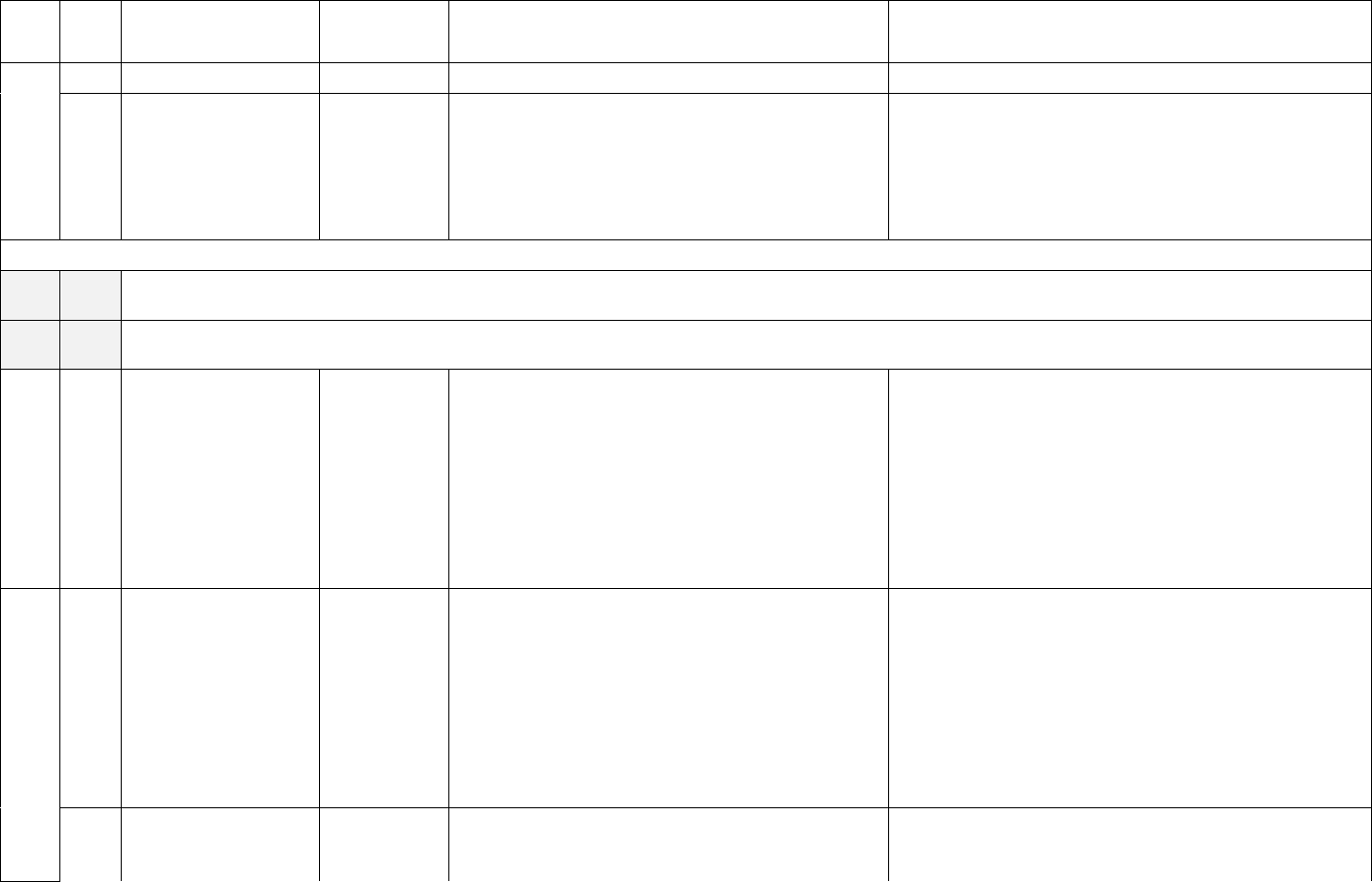
10
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
week
1
h.
Optional
Weekly, for
All days in
previous
week
HHS ID
The HHS-assigned facility ID. If multiple facilities
report under the same CCN, each individual facility
will have a unique HHS ID. See Appendix D for
additional information.
Capacity, Occupancy, Hospitalizations, Admissions
2
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (All hospital beds)
2
b.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (All adult hospital beds)
3
a.
Required
Weekly, for
All days in
previous
week
All hospital inpatient beds
Total number of all staffed inpatient beds in the
facility, that are currently set-up, staffed and able to be
used for a patient within the reporting period. This
includes all overflow, observation, and active
surge/expansion beds used for inpatients. This
includes ICU beds. Include any
surge/hallway/overflow beds that are open for use for
a patient, regardless of whether they are occupied or
available.
3
b.
Required
Weekly, for
All days in
previous
week
Adult hospital inpatient beds (Subset)
Total number of all staffed adult inpatient beds in the
facility, that are currently set-up, staffed and able to be
used for a patient within the reporting period. This
includes all overflow, observation, and active
surge/expansion beds used for inpatients. This
includes ICU beds. Include any
surge/hallway/overflow beds that are open for use for
a patient, regardless of whether they are occupied or
available. This is a subset of #3a.
3
c.
Required
Weekly, for
All days in
All inpatient pediatric beds (Subset)
Total number of pediatric beds in the facility that are
currently set-up, staffed and able to be used for a
patient within the reporting period. This count
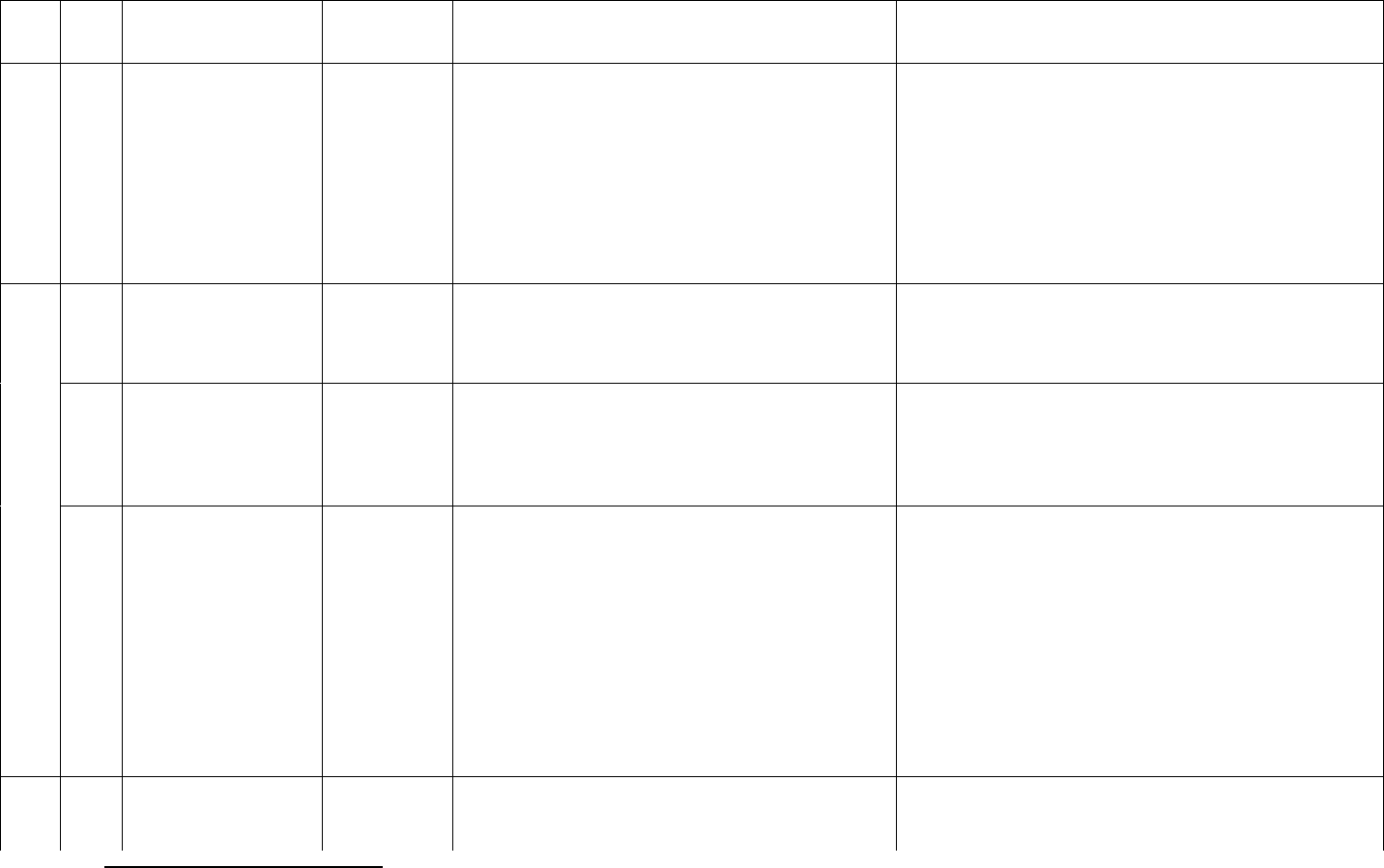
11
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
previous
week
includes occupied and unoccupied inpatient pediatric
beds including both PICU and med-surge beds (beds
in which medical or surgical pediatric patients may be
routinely placed). Include any surge/hallway/overflow
beds that are open for use for a patient, regardless of
whether they are occupied or available. This count
excludes NICU, newborn nursery beds, and
outpatient surgery beds. This is a subset of #3a. This
field is required as of 2/2/2022.
4
a.
Required
Weekly, for
All days in
previous
week
All hospital inpatient bed occupancy
Total number of staffed inpatient beds that are
occupied. This reflects occupancy levels for beds
reported in #3a.
4
b.
Required
Weekly, for
All days in
previous
week
Adult hospital inpatient bed occupancy (Subset)
Total number of staffed adult inpatient beds that are
occupied. This is a subset of #4a, and reflects
occupancy levels for beds reported in #3b.
4
c.
Required
Weekly, for
All days in
previous
week
Pediatric inpatient bed occupancy (Subset)
Total number of set-up and staffed inpatient pediatric
beds that are occupied by a patient. Includes both
PICU and med-surge beds (beds in which medical or
surgical pediatric patients may be routinely placed).
Include any occupied surge/hallway/overflow beds
that are open for use. This count excludes NICU,
newborn nursery, and outpatient surgery beds
unless they are beds designated for COVID-19
positive pediatric patients. This is a subset of #4a,
and reflects occupancy levels for beds reported in #3c.
This field is required as of 2/2/2022.
5
4
a.
Required
Weekly, for
All days in
previous
ICU beds (Subset)
Total number of ICU beds that are currently set-up,
staffed and are or could be used for a patient within
the reporting period. This count includes occupied and
4
Data collection systems are encouraged to provide mechanisms for hospitals without ICUs to skip all ICU questions.
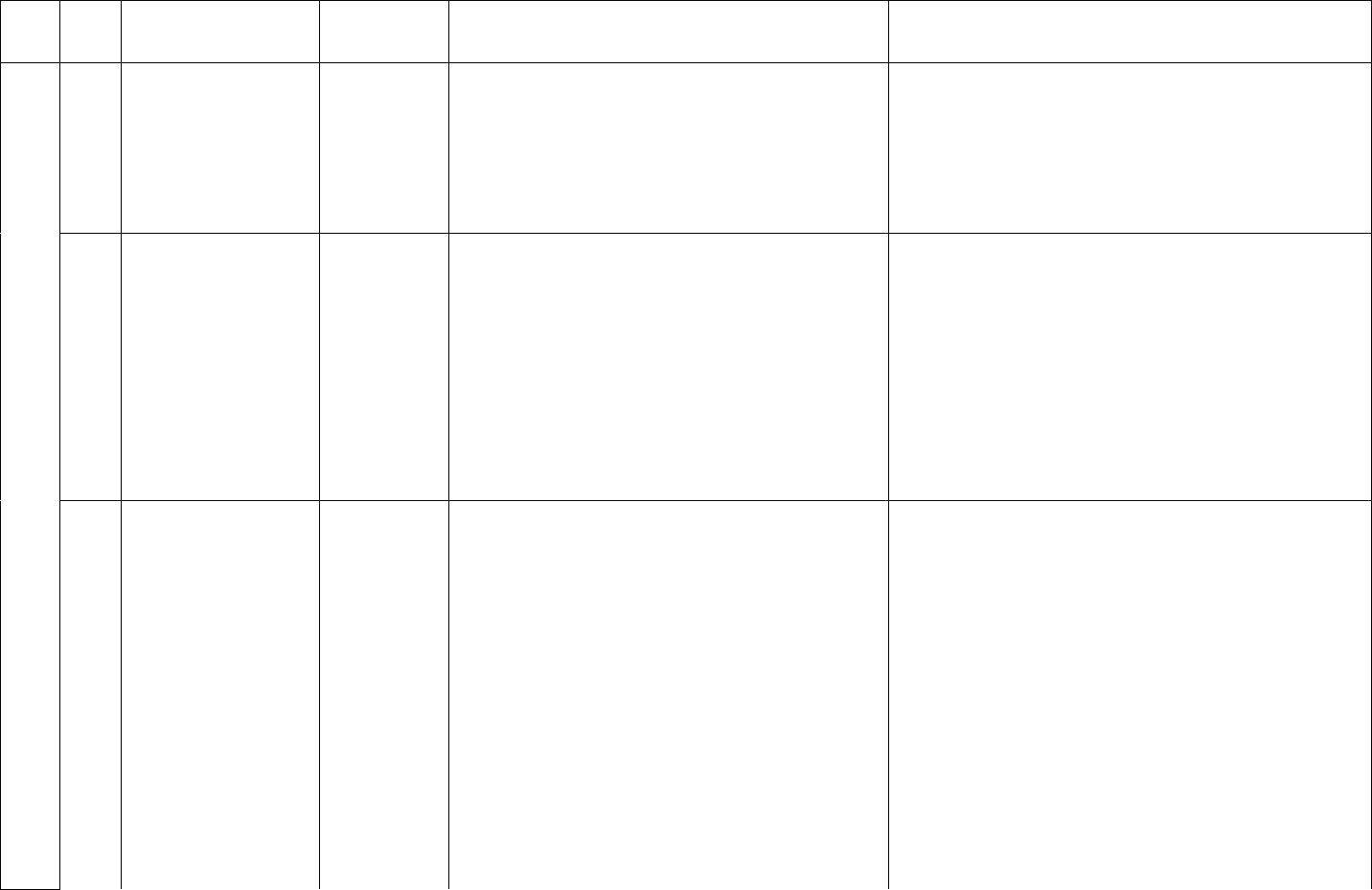
12
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
week
unoccupied ICU beds. This is a subset of #3a, and
includes the values for #5b and #5c.
Note: All ICU beds should be considered, regardless
of the unit on which the bed is housed. This includes
ICU beds located in non-ICU locations, such as mixed
acuity units.
5
b.
Required
Weekly, for
All days in
previous
week
Adult ICU beds (Subset)
Total number of staffed adult inpatient ICU beds that
are currently set-up, staffed and are or could be used
for a patient within the reporting period. This count
includes occupied and unoccupied ICU beds. This is
a subset of #3b and #5a. Any beds counted in #5b
should NOT be counted in #5c.
Note: All adult ICU beds should be considered,
regardless of the unit on which the bed is housed. This
includes ICU beds located in non-ICU locations, such
as mixed acuity units.
5
c.
Required
Weekly, for
All days in
previous
week
Pediatric ICU beds (Subset)
Total number of pediatric ICU beds in the facility that
are currently set-up, staffed and are or could be used
for a patient within the reporting period. This count
includes occupied and unoccupied ICU beds,
including any ICU beds that are, or could be, staffed
and used for a pediatric patient. This count excludes
NICU, newborn nursery, and outpatient surgery
beds unless they are beds designated for COVID-
19 positive pediatric patients. This is a subset of #3c
and #5a. Any beds counted in #5c should NOT be
counted in #5b. This field is required as of 2/2/2022.
Note: All pediatric ICU beds should be considered,
regardless of the unit on which the bed is housed. This
includes ICU beds located in non-ICU locations, such
as mixed acuity units.
13
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
6
a.
Required
Weekly, for
All days in
previous
week
ICU bed occupancy (Subset)
Total number of staffed ICU beds that are occupied.
This is a subset of #4a.
6
b.
Required
Weekly, for
All days in
previous
week
Adult ICU bed occupancy (Subset)
Total number of staffed adult ICU beds that are
occupied. This is a subset of #4b and #6a.
6
c.
Required
Weekly, for
All days in
previous
week
Pediatric ICU bed occupancy (Subset)
Total number of set-up and staffed pediatric ICU beds
occupied by a patient. This count excludes NICU,
newborn nursery, and outpatient surgery beds
unless they are beds designated for COVID-19
positive pediatric patients. This is subset of #4c and
#6a. This field is required as of 2/2/2022.
Note: All occupied pediatric ICU beds should be
considered, regardless of the unit on which the bed is
housed. This includes ICU beds located in non-ICU
locations, such as mixed acuity units.
7
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Total mechanical ventilators)
8
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Mechanical ventilators in use)
9
a.
Optional
Weekly, for
All days in
previous
week
Total hospitalized adult suspected or laboratory
confirmed COVID-19 patients
Patients currently hospitalized in an adult inpatient
bed who have laboratory-confirmed or suspected
COVID-19. Include those in observation beds.
14
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
See Appendix D for the definition of laboratory-
confirmed COVID-19.
9
b.
Required
Weekly, for
All days in
previous
week
Hospitalized adult laboratory-confirmed
COVID-19 patients
Patients currently hospitalized in an adult inpatient
bed who have laboratory-confirmed COVID-19.
Include those in observation beds. Include patients
who have both laboratory-confirmed COVID-19 and
laboratory-confirmed influenza in this field.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
10
a.
Optional
Weekly, for
All days in
previous
week
Total hospitalized pediatric suspected or
laboratory-confirmed COVID-19 patients
Patients currently hospitalized in a pediatric inpatient
bed, including NICU, PICU, newborn, and nursery,
who are suspected or laboratory-confirmed-positive
for COVID-19. Include those in observation beds.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
10
b.
Required
Weekly, for
All days in
previous
week
Hospitalized pediatric laboratory-confirmed
COVID-19 patients
Patients currently hospitalized in a pediatric inpatient
bed, including NICU, PICU, newborn, and nursery,
who have laboratory-confirmed COVID-19. Include
those in observation beds. Include patients who have
both laboratory-confirmed COVID-19 and laboratory-
confirmed influenza in this field.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
11
Optional
Weekly, for
All days in
previous
week
Hospitalized and ventilated COVID-19 patients
Patients currently hospitalized in an adult, pediatric, or
neonatal inpatient bed who have suspected or
laboratory-confirmed COVID-19 and are on a
mechanical ventilator including adult, pediatric,
neonatal ventilators, ECMO machines, anesthesia
machines and portable/transport ventilators available
in the facility. Include BiPAP machines if the hospital
15
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
uses BiPAP to deliver positive pressure ventilation via
artificial airways.
12
a.
Optional
Weekly, for
All days in
previous
week
Total ICU adult suspected or laboratory-
confirmed COVID-19 patients
Patients currently hospitalized in a designated adult
ICU bed who have suspected or laboratory-confirmed
COVID-19.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
12
b.
Required
Weekly, for
All days in
previous
week
Hospitalized ICU adult laboratory-confirmed
COVID-19 patients
Patients currently hospitalized in an adult ICU bed
who have laboratory-confirmed COVID-19. Include
patients who have both laboratory-confirmed COVID-
19 and laboratory-confirmed influenza in this field.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
12
c.
Required
Weekly, for
All days in
previous
week
Hospitalized ICU pediatric laboratory-confirmed
COVID-19 patients
Total number of pediatric ICU beds occupied by
laboratory confirmed positive COVID-19 patients.
This is a subset of #6c, occupied pediatric ICU beds.
This count excludes NICU, newborn nursery, and
outpatient surgery beds unless they are beds
designated for COVID-19 positive pediatric
patients. This field is required as of 2/2/2022.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
13
Optional
Weekly, for
All days in
previous
week
Hospital Onset
Total current inpatients with onset of suspected or
laboratory-confirmed COVID-19 fourteen or more
days after admission for a condition other than
COVID-19.
14
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (ED/overflow)
16
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
15
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (ED/overflow and ventilated)
16
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Previous day’s COVID-19 deaths)
17
a.
Required
Weekly, for
All days in
previous
week
Previous day’s adult admissions with laboratory-
confirmed COVID-19 and breakdown by age
bracket:
• 18-19
• 20-29
• 30-39
• 40-49
• 50-59
• 60-69
• 70-79
• 80+
• Unknown
Enter the number of patients by age bracket who were
admitted to an adult inpatient bed on the previous
calendar day who had laboratory-confirmed COVID-
19 at the time of admission. This is a subset of #9b.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
b.
Optional
Weekly, for
All days in
previous
week
Previous day’s adult admissions with suspected
COVID-19 and breakdown by age bracket:
• 18-19
• 20-29
• 30-39
• 40-49
• 50-59
• 60-69
• 70-79
• 80+
• Unknown
Enter the number of patients by age bracket who were
admitted to an adult inpatient on the previous calendar
day who had suspected COVID-19 at the time of
admission. This is a subset of #9a.
18
a.
Required
Weekly, for
All days in
previous
week
Previous day’s pediatric admissions with
laboratory-confirmed COVID-19
Enter the number of pediatric patients (patients 0 – 17
years old) who were admitted to an inpatient bed
(regardless of whether the bed is designated as
pediatric vs adult), including NICU, PICU, newborn,
and nursery, on the previous calendar day who had
17
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
laboratory-confirmed COVID-19 at the time of
admission.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
b.
Optional
Weekly, for
All days in
previous
week
Previous day’s pediatric admissions with
suspected COVID-19
Enter the number of pediatrics patients (patients 0 –
17 years old) who were admitted to an inpatient bed
(regardless of whether the bed is designated as
pediatric vs adult), including NICU, PICU, newborn,
and nursery, on the previous calendar day who had
suspected COVID-19 at the time of admission. This is
a subset of #10a.
18
c.
Required
Weekly, for
All days in
previous
week
Previous day’s pediatric admissions with
laboratory-confirmed COVID-19 breakdown by
age group:
• 0-4
• 5-11
• 12-17
• Unknown
Enter the number of patients, by age group, who were
admitted to an inpatient or ICU bed on the previous
calendar day who had laboratory-confirmed COVID-
19 at the time of admission. The summary of age
breakdowns should be identical to #18a.
This includes patients ages 0-4, 5-11, and 12-17 years
old admitted to any inpatient bed, regardless of
whether the bed is designated as pediatric vs. adult.
This field is required as of 2/2/2022.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
19
Optional
Weekly, for
All days in
previous
week
Previous day’s Emergency Department (ED)
Visits
Enter the total number of patient visits to the ED who
were seen on the previous calendar day regardless of
reason for visit. Include all patients who are triaged
even if they leave before being seen by a provider.
18
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
20
Optional
Weekly, for
All days in
previous
week
Previous day’s total COVID-19- related ED
visits (Subset)
Enter the total number of ED visits who were seen on
the previous calendar day who had a visit related to
suspected or laboratory-confirmed COVID-19.
Do not count patients who receive a COVID-19 test
solely for screening purposes in the absence of
COVID-19 symptoms.
“Suspected” is defined as a person who is being
managed as though he/she has COVID-19 because of
signs and symptoms suggestive of COVID-19 but
does not have a laboratory-positive COVID-19 test
result.
See Appendix D for the definition of laboratory-
confirmed COVID-19.
21
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Previous day’s remdesivir used)
22
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Current inventory of remdesivir)
23
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Critical staffing shortage today (Y/N)
24
Optional
Weekly
+
, for
Wednesday
collection
date
Critical staffing shortage anticipated within a
week (Y/N)
Enter Y if you anticipate a critical staffing shortage
within a week. Enter N if you do not anticipate a
staffing shortage within a week. If you do not report
this value, the default is N. If you have a shortage,
report Y until the shortage is resolved.
Each facility should identify staffing shortages based
on their facility needs and internal policies for staffing
ratios. The use of temporary staff does not count as a
staffing shortage if staffing ratios are met according to
the facility’s needs and internal policies for staffing
ratios.
19
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
25
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Additional details)
Supplies
Note: Supply reporting is NOT intended to replace request for resources processes.
26
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Are your PPE supply items managed at the facility level or centrally)
27
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (On hand Ventilator Supplies)
27
b.
Required
Weekly
+
,for
Wednesday
collection
date
On hand supply duration in days: N95
respirators
Provide calculated range of days of supply in stock for
each PPE category. For supply categories that may
have varying quantities or days on hand, report the
days on hand for the item that has the lowest stock on
hand.
• 0 days
• 1-3 days
• 4-6 days
• 7-14 days
• 15-30 days
• >30 days
Calculations may be provided by your hospital’s ERP
system or by utilizing the CDC’s PPE burn rate
calculator assumptions.
27
c.
Required
Weekly
+
,
for
Wednesday
collection
date
On hand supply duration in days: Surgical and
procedure masks
27
d.
Required
Weekly
+
,
for
Wednesday
On hand supply duration in days: Eye protection
including face shields and goggles
20
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
collection
date
27
e.
Required
Weekly
+
,
for
Wednesday
collection
date
On hand supply duration in days: Single-use
gowns
27
f.
Required
Weekly
+
,
for
Wednesday
collection
date
On hand supply duration in days: Exam gloves
(sterile and non-sterile)
28
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, n95 respirators)
28
b.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, other respirators)
28
c.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, surgical and procedural masks)
28
d.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, eye protection)
28
e.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, single use gowns)
28
f.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, launderable gowns)
28
g.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Eaches, exam gloves)
29
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to obtain, ventilator supplies)
29
b.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to obtain, ventilator medications)
21
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
29
c.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to obtain, n95s)
29
d.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to obtain, other respirators)
29
e.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to obtain, surgical and procedural masks)
29
f.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to Obtain, eye protection)
29
g.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to Obtain, single use gowns)
29
h.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to Obtain, exam gloves)
29
i.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Able to maintain supply of launderable gowns)
30
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, ventilator supplies)
30
b.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, ventilator medications)
30
c.
Required
Weekly
+
,
for
Wednesday
collection
date in
previous
week
Are you able to maintain at least a 3-day supply
of N95 respirators?
(Y, N, N/A) Enter Y if your facility is able to maintain
at least a 3-day supply of N95 respirators. Enter N if
your facility is not able to maintain at least a 3-day
supply of N95 respirators. Enter N/A if N95
respirators are not relevant at your facility.
30
d.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, other respirators)
30
e.
Required
Weekly
+
,
for
Wednesday
collection
date in
Are you able to maintain at least a 3-day supply
of surgical and procedural masks?
(Y, N, N/A) Enter Y for each supply type for which
your facility is able to maintain at least a 3-day
supply. Enter N for those supply types your facility is
not able to maintain at least a 3-day supply. Enter N/A
for each supply type that is not relevant at your
facility.
22
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
previous
week
30
f.
Required
Weekly
+
,
for
Wednesday
collection
date in
previous
week
Are you able to maintain at least a 3-day supply
of eye protection including face shields and
goggles?
30
g.
Required
Weekly
+
,
for
Wednesday
collection
date in
previous
week
Are you able to maintain at least a 3-day supply
of single-use gowns?
30
h.
Required
Weekly
+
,
for
Wednesday
collection
date in
previous
week
Are you able to maintain at least a 3-day supply
of exam gloves?
30
i.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, nasal pharyngeal swabs)
30
j.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, nasal swabs)
30
k.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Maintain, viral transport media)
23
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
31
a.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Reuse gowns)
31
b.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Reuse PAPRS)
31
c.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Reuse n95)
32
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal government. No
change is required to reporting templates. (Additional details)
Influenza
33
Required
Weekly, for
all days in
the previous
week in
previous
week
Total hospitalized patients with laboratory-
confirmed influenza virus infection
Enter the total number of patients (adult and pediatric)
currently hospitalized in an inpatient bed who have
laboratory-confirmed influenza virus infection.
Include inpatient, overflow, observation, ED, ED
awaiting orders for an inpatient bed, active
surge/expansion, ICU, NICU, PICU, newborn and
nursery. This field is required as of 2/2/2022.
See Appendix D for the definition of laboratory-
confirmed influenza.
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized adult patients with laboratory-
confirmed influenza virus infection
[NEW]
Patients currently hospitalized in an adult inpatient
bed who have laboratory-confirmed influenza. Include
those in observation beds. Include patients who have
laboratory-confirmed RSV and/or COVID-19 and/or
laboratory-confirmed influenza in this field
(coinfections).
See Appendix D for the definition of laboratory-
confirmed influenza.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
[NEW]
Hospitalized pediatric patients with laboratory-
confirmed influenza virus infection
[NEW]
Patients currently hospitalized in a pediatric inpatient
bed, including NICU, PICU, newborn, and nursery,
who have laboratory-confirmed influenza virus
24
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
week in
previous
week
infection. Include those in observation beds. Include
patients who have laboratory-confirmed RSV and/or
COVID-19 and/or laboratory-confirmed influenza in
this field (coinfections).
See Appendix D for the definition of laboratory-
confirmed influenza.
34
Required
Weekly, for
all days in
the previous
week in
previous
week
Previous day’s admissions with laboratory-
confirmed influenza virus infection
Enter the total number of patients (adult and pediatric)
who were admitted to an inpatient bed on the previous
calendar day who had laboratory-confirmed influenza
virus infection at the time of admission. Include
inpatient, overflow, observation, ED, ED awaiting
orders for an inpatient bed, active surge/expansion,
ICU, NICU, PICU, newborn and nursery. This field is
required as of 2/2/2022.
See Appendix D for the definition of laboratory-
confirmed influenza.
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Previous day’s adult admissions with laboratory-
confirmed influenza virus infection
[NEW]
Enter the total number of adult patients (age 18 and
older) who were admitted to an adult inpatient bed on
the previous calendar day who had laboratory-
confirmed influenza infection at the time of
admission. Include inpatient, overflow, observation,
ED, ED awaiting orders for an inpatient bed, active
surge/expansion, ICU.
See Appendix D for the definition of laboratory-
confirmed influenza.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
[NEW]
Previous day’s pediatric admissions with
laboratory-confirmed influenza virus infection
[NEW]
Enter the number of pediatric patients (patients 0 – 17
years old) who were admitted to an inpatient bed
(regardless of whether the bed is designated as
25
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
week in
previous
week
pediatric vs adult), including NICU, PICU, newborn,
and nursery, on the previous calendar day who had
laboratory-confirmed influenza at the time of
admission.
See Appendix D for the definition of laboratory-
confirmed influenza.
35
Required
Weekly, for
all days in
the previous
week in
previous
week
Total hospitalized ICU patients with laboratory-
confirmed influenza virus infection
Enter the total number of patients (adult and pediatric)
currently hospitalized in a designated ICU bed with
laboratory-confirmed influenza virus infection. This is
a subset of #33—this value should not exceed the
value in #33. This field is required as of 2/2/2022.
See Appendix D for the definition of laboratory-
confirmed influenza.
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized ICU adult laboratory-confirmed
influenza patients
[NEW]
Patients currently hospitalized in an adult ICU bed
who have laboratory-confirmed influenza. Include
patients who have laboratory-confirmed RSV
and/or COVID-19, and/or laboratory-confirmed
influenza in this field (coinfections)
See Appendix D for the definition of laboratory-
confirmed influenza.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized ICU pediatric laboratory-confirmed
influenza patients
[NEW]
Total number of pediatric ICU beds occupied by
laboratory confirmed influenza patients. This is a
subset of #6c, occupied pediatric ICU beds.
See Appendix D for the definition of laboratory-
confirmed influenza.
26
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
36
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Total hospitalized patients co- infected with both laboratory-confirmed COVID-19
and laboratory-confirmed influenza virus infection)
37
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Previous day’s influenza deaths (laboratory-confirmed influenza virus infection)
38
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Previous day’s deaths for patients co-infected with both COVID-19 AND laboratory-
confirmed influenza virus)
Therapeutics
As of November 2, 2022, therapeutic data are reported to the Healthcare Provider Ordering Portal (HPOP) system,. This change consolidated therapeutic
reporting for all products and ordering in one location. Please note, the data elements and/or reporting cadence may be adjusted based on therapeutic team
needs. Please follow HPOP reporting guidance starting November 2, 2022. Prior to November 2, 2022, the therapeutic data elements were required for
reporting to the Unified Hospital Data Surveillance System once weekly on Wednesdays.
39
a.
This field was moved to HPOP on November 2, 2022. (Therapeutic A, Casirivimab/Imdevimab, Courses on Hand)
39
b.
This field was moved to HPOP on November 2, 2022. (Therapeutic A, Casirivimab/Imdevimab, Courses Administered in Last Week)
39
c.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Therapeutic B On Hand)
39
d.
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government. No change is required to reporting templates. (Therapeutic B Courses Administered)
40
a.
This field was moved to HPOP on November 2, 2022. (Therapeutic C, Bamlanivimab/Etsevimab), Courses on Hand)
40
b.
This field was moved to HPOP on November 2, 2022. (Therapeutic C, Bamlanivimab/Etsevimab, Courses Administered in Last Week)
40
c.
This field was moved to HPOP on November 2, 2022. (Therapeutic D, Sotrovimab, Courses on Hand)
40
d.
This field was moved to HPOP on November 2, 2022. (Therapeutic D, Sotrovimab, Courses Administered in Last Week)
Therapeutic Placeholders
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
e.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
f.
As of August 10, 2022, therapeutic placeholders are being made inactive due to all therapeutic reporting being moved into HPOP on November
2, 2022.
40
g.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.

27
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
40
h.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
i.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
j.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
k.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
l.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
m.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
n.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
o.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
40
p.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2, 2022.
Healthcare Worker Vaccination
As of August 10, 2022, healthcare worker vaccination fields are federally inactive within the Unified Hospital Data Surveillance System. As a reminder, CMS
rule CMS-1752-F and CMS-1762-F requires hospital worker vaccination rates to be reported on a regular basis into the National Healthcare Safety Network
(NHSN) as a quality measure beginning on October 1, 2021. NHSN has provided additional information and resources on the measures being collected. The
below vaccination data elements below are inactive for federal collection and do NOT meet the requirements of the CMS rule.
41
This field is inactive for COVID-19 hospital data collection in the NHSN Patient Safety Component, COVID-19 Hospital Data Module. Please
ensure complete reporting to NHSN Healthcare Personnel Safety Component per CMS guidance.
42
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Current healthcare personnel, no COVID-19 vaccine doses)
43
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Current healthcare personnel, first COVID-19 vaccine dose)
28
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
44
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Current healthcare personnel, completed COVID-19 vaccine series)
45
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Total current healthcare personnel)
46
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Patient, first COVID-19 vaccine dose)
47
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. No change
is required to reporting templates. (Patient, completed COVID-19 vaccine series)
Respiratory Syncytial Virus (RSV)
48
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized adult patients with laboratory-
confirmed RSV infection
[NEW]
Patients currently hospitalized in an adult inpatient
bed who have laboratory-confirmed RSV. Include
those in observation beds. Include patients who have
laboratory-confirmed RSV and/or COVID-19 and/or
laboratory-confirmed influenza in this field
(coinfections).
See Appendix D for the definition of laboratory-
confirmed RSV.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized pediatric patients with laboratory-
confirmed RSV infection
[NEW]
Patients currently hospitalized in a pediatric
inpatient bed, including NICU, PICU,
newborn, and nursery, who have laboratory
confirmed RSV. Include those in observation beds.
Include patients who have laboratory-confirmed
RSV and/or COVID-19 and/or laboratory
confirmed influenza in this field (coinfections).
See Appendix D for the definition of laboratory-
confirmed RSV.
29
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
49
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Previous day’s adult admissions with laboratory-
confirmed RSV infection
[NEW]
Enter the total number of adult patients (age 18 and
older) who were admitted to an adult inpatient bed on
the previous calendar day who had laboratory-
confirmed RSV infection at the time of admission.
Include inpatient, overflow, observation, ED, ED
awaiting orders for an inpatient bed, active
surge/expansion, ICU.
See Appendix D for the definition of laboratory-
confirmed RSV.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Previous day’s pediatric admissions with
laboratory-confirmed RSV infection
[NEW]
Enter the number of pediatric patients (patients 0
– 17 years old) who were admitted to an inpatient
bed (regardless of whether the bed is designated as
pediatric vs adult), including NICU, PICU,
newborn, and nursery, on the previous calendar
day who had laboratory-confirmed RSV at the
time of admission.
See Appendix D for the definition of laboratory-
confirmed RSV.
50
a.
[NEW]
Optional
[NEW]
Weekly, for
all days in
the previous
week in
previous
week
[NEW]
Hospitalized ICU adult laboratory-confirmed
RSV patients
[NEW]
Patients currently hospitalized in an adult ICU bed
who have laboratory-confirmed RSV. Include patients
who have laboratory-confirmed RSV and/or COVID-
19, and/or laboratory-confirmed influenza in this field
(coinfections).
See Appendix D for the definition of laboratory-
confirmed RSV.
b.
[NEW]
Optional
[NEW]
Weekly, for
all days in
[NEW]
Hospitalized ICU pediatric laboratory-confirmed
RSV patients
[NEW]

30
ID
Sub
ID
Required/Optional
Reporting
Cadence
Information Needed
Description
the previous
week in
previous
week
Total number of pediatric ICU beds occupied by
laboratory confirmed positive RSV patients. This is a
subset of #6c, occupied pediatric ICU beds.
See Appendix D for the definition of laboratory-
confirmed RSV.
* indicates information should be provided daily for each day in the previous week, NOT aggregated to weekly values
+indicates information should be provided once a week on Wednesdays
Therapeutic Data Elements
On November 2, 2022, therapeutic data reporting transitioned to the Health Partner Ordering Portal (HPOP). HPOP is an ordering portal for
requesting COVID-19 therapeutic products provided at no cost by the Administration for Strategic Preparedness and Response within the U.S.
Department of Health and Human Services. HPOP is used to order COVID-19 therapeutic products allocated by HHS/ASPR. Further information
will be provided on the therapeutic reporting transition to HPOP, with the therapeutic team determining reporting needs accordingly. While
therapeutic data will not be reported to NHSN or included in Unified Hospital Data Surveillance System reporting as of November 2, 2022,
therapeutic data remains important to the federal response. This information is needed for tracking purposes and strategic decision making. All
hospitals should follow reporting requirements through HPOP. Questions on therapeutic data reporting can be directed to:
Testing Data Elements: Hospitals Performing COVID-19 Testing Using an In-House Laboratory
Effective May 11, 2023, HHS announced the Public Health Emergency declaration for COVID-19 has ended. In addition, HHS and CLIA
regulatory requirements under the CARES Act Authority requiring reporting of laboratory test data to the federal government have also ended.
State-specific reporting requirements may still apply. Laboratories and testing sites should review state-specific reporting requirements for
COVID-19 testing. They may be required to continue reporting for COVID-19 testing to their State Health Departments.
Hospital Data Usage & Access
[CHANGE] Hospital data are collated, manipulated, and visualized at the Federal level in these primary locations: internally, via CDC’s National
Healthcare Safety Network (NHSN) and HHS Protect, and externally on CDC public webpages including COVID Data Tracker (CDT) and
data.cdc.gov.

31
HHS Protect serves as an internal hub for data analysis and visualization, allowing integration of additional datasets from other sources. Federal
decision-makers and analysts can access the data through HHS Protect directly, or indirectly through various generated reports. A variety of
Federal teams use the data as detailed in the above data element table. In addition to Federal partners, state, tribal, local, and territorial partners
also have access to the data through HHS Protect. Tribal partners are encouraged to work with the Indian Health Service (IHS) and respective state
partners to define geographical access accordingly. HHS regional staff, ASPR regional staff and/or Indian Health Service (IHS) staff serve as
HHS Protect sponsors for respective state, tribe, and territory users. Local partners also have access to the data, working in conjunction with their
respective state to define geographical access accordingly. State HHS Protect users serve as sponsors for local partners. Organizations, such as
hospital associations, can be provided access to the data if granted written permission by the state and/or an individual reporting hospital facility.
To inquire about an HHS Protect account, email the Protect Service Desk (hhsprotect@cdc.gov).
Information within HHS Protect is secured through robust usage and access controls. All users must be sponsored to gain access to HHS Protect
by the mechanisms mentioned above. All data have accompanying sharing and use agreements, specifying how and with whom the information
can be exported and shared.
As of February 2023, HHS Protect Public is no longer available. This site served as a fully public data hub, providing aggregated content and
dashboards. HHS Protect Public contained aggregated subsets of the hospital data, providing transparency for all stakeholders. More information is
listed on the banner on the HHS Protect Public webpage: https://protect-public.hhs.gov/.
Hospital Data Quality & Errors
Quality data helps to ensure informed decision-making based on accurate information. Federal partners regularly conduct data quality checks, and
may contact state and territorial partners if further information is needed. Federal partners will not contact facilities directly unless explicitly
granted permission by the state and/or in extraordinary circumstances.
Hospital data liaisons work collaboratively with state and territorial partners to increase transparency, as well as verify and resolve any data
challenges. Importantly, data liaisons work specifically with data. Operational needs and resource requests for personnel, supplies, technical
assistance, and/or other needs follow all normal processes and should NOT be directed to hospital data liaisons.
Users who identify any errors in their data are encouraged to contact the NHSN Help Desk ([email protected]).

32
Appendix A: Change Log
The change log details changes in the hospital reporting guidance to aid partners in tracking updates.
[CHANGE] Changes from the June 11, 2023 COVID-19 Hospital Reporting Guidance and FAQs (dated
November 17, 2023)
Data Element Changes
The following changes were made to hospital reporting data elements:
• New Data Elements Added (all optional):
o 33a. Hospitalized adult patients with laboratory-confirmed influenza virus infection
o 33b. Hospitalized pediatric patients with laboratory-confirmed influenza virus infection
o 34a. Previous day’s adult admissions with laboratory-confirmed influenza virus infection
o 34b. Previous day’s pediatric admissions with laboratory-confirmed influenza virus infection
o 35a. Hospitalized ICU adult laboratory-confirmed influenza patients
o 35b. Hospitalized ICU pediatric laboratory-confirmed influenza patients
o 48a. Hospitalized adult laboratory-confirmed RSV patients
o 48b. Hospitalized pediatric laboratory-confirmed RSV patients
o 49a. Previous day’s adult admissions with laboratory-confirmed RSV
o 49b. Previous day’s pediatric admissions with laboratory-confirmed RSV
o 50a. Hospitalized ICU adult laboratory-confirmed RSV patients
o 50b. Hospitalized ICU pediatric laboratory-confirmed RSV patients
Narrative and FAQ Changes
• The narrative and FAQs were streamlined and reorganized for clarity.
• Additional introductory information was added for clarification and describing the purpose of expanding the data collection to include
additional optional respiratory pathogen reporting.
33
Changes from the December 15, 2022 COVID-19 Hospital Reporting Guidance and FAQs (dated June 11,
2023)
Cadence Changes
As of the June 11, 2023 guidance, all hospitals (except psychiatric and rehabilitation hospitals) are required to data report once weekly to NHSN.
The weekly data submission should be submitted by Tuesday, 1159pm local time, and still include daily data for each day in the previous week,
defined as the previous Sunday through Saturday.
Data should NOT be aggregated to weekly values. It is critical that the data are reported by Tuesday in order to count towards compliance. For
items that were previously reported once per week, data for Wednesday during the reporting week should be included to count towards
compliance requirements.
Data Element Changes
• Data elements made optional:
o 17b: Previous day’s adult admissions with suspected COVID-19, total
o 17b-1: Previous day’s adult admissions with suspected COVID-19, 18-19 years
o 17b-2: Previous day’s adult admissions with suspected COVID-19, 20-29 years
o 17b-3: Previous day’s adult admissions with suspected COVID-19, 30-39 years
o 17b-4: Previous day’s adult admissions with suspected COVID-19, 40-49 years
o 17b-5: Previous day’s adult admissions with suspected COVID-19, 50-59 years
o 17b-6: Previous day’s adult admissions with suspected COVID-19, 60-69 years
o 17b-7: Previous day’s adult admissions with suspected COVID-19, 70-79 years
o 17b-8: Previous day’s adult admissions with suspected COVID-19, 80+ years
o 17b-9: Previous day’s adult admissions with suspected COVID-19, unknown
o 18b: Previous day’s pediatric admissions with suspected COVID-19
o 9a: Total hospitalized adult suspected or laboratory confirmed COVID-19 patients
o 10a: Total hospitalized pediatric suspected or laboratory-confirmed COVID-19 patients
o 11: Hospitalized and ventilated COVID-19 patients
o 12a: Total ICU adult suspected or laboratory-confirmed COVID-19 patients
o 13: Hospital onset
o 19: Previous day’s Emergency Department (ED) visits
o 20: Previous day’s total COVID-19- related ED visits (Subset)

34
Narrative and FAQ Changes
• The narrative and FAQs were streamlined and reorganized for clarity.
Changes from the August 15, 2022 COVID-19 Hospital Reporting Guidance and FAQs (dated December 15,
2022)
The only changes made in the December 15, 2022 version are updates related to the transition of reporting to the National Healthcare Safety
Network (NHSN). There are no significant changes or additions to the reporting questions as a result of this transition. Reporting
requirements will remain the same, with the only significant change being the data system and the need to use NHSN orgIDs. Information on the
transition is available on the transition website, located at https://www.cdc.gov/nhsn/covid19/transition.html.
Data Element Changes
• NHSN orgID is now needed to submit data
Changes from the previous COVID-19 Hospital Reporting Guidance and FAQs (dated January 6, 2022)
Facility Type Changes
• Psychiatric & Rehabilitation Hospitals: Per Secretary discretion, psychiatric and rehabilitation facility federal reporting has been set to
submitting data once annually from October to October. This may evolve based on needs of the national response. See Appendix D for
additional details on reporting.
Data Element Changes
Several data elements have been made inactive for federal collection within the Unified Hospital Data Surveillance System (UHDSS). Some data
elements, – while inactive or soon to be inactive in UHDSS – are being collected through other mechanisms, including vaccination and therapeutic
data. These changes have been marked as [CHANGE] and highlighted in orange. Changes from previous guidance updates are no longer marked
as changes.

35
On November 2, 2022, data elements related to therapeutics will be moved for collection in the Healthcare Provider Ordering Portal
(HPOP).These data elements include:
• 39a: Therapeutic A courses on hand
• 39b: Therapeutic A courses administered
• 40a: Therapeutic C courses on hand
• 40b: Therapeutic C courses administered
• 40c: Therapeutic D courses on hand
• 40d: Therapeutic D courses administered
• 40e-p: Therapeutic placeholders
Healthcare worker vaccination fields have been made federally inactive within the Unified Hospital Data Surveillance System. As a reminder,
CMS rule CMS-1752-F and CMS-1762-F requires hospital worker vaccination rates to be reported on a regular basis into the National Healthcare
Safety Network (NHSN) as a quality measure beginning on October 1, 2021.
• 41: Vaccine doses administered to healthcare personnel
• 42: Healthcare personnel, no COVID-19 vaccine
• 43: Healthcare personnel, first COVID-19 vaccine in series
• 44: Healthcare personnel, completed COVID-19 vaccine series
• 45: Total number of healthcare personnel
• 46: Patients, first COVID-19 vaccine in series
• 47: Patients, completed COVID-19 vaccine in series
Narrative Changes
• Added information on therapeutic move to HPOP.
• Added information on psychiatric and rehabilitation facility reporting in narrative and Appendix D.
• Removed therapeutic calculator from Appendix D.
• Clarifications added to narrative and/or data element notes based clarifications previously issued to date.
Changes from earlier COVID-19 Hospital Reporting Guidance and FAQs (dated May 27, 2020)
Numerous changes were implemented in the latest version of the hospital reporting guidance. To help users to navigate changes quickly, changes
are grouped based on the following areas: cadence and facility type changes; data element changes; laboratory data element changes; and narrative
and FAQ changes.

36
Cadence and Facility Type Changes
• Flexibilities on data reporting on weekends and holidays were clarified.
• Information describing facility types was reformatted for clarity.
Data Element Changes
The following changes were made to hospital reporting data elements:
• New Data Elements Added:
o 1h: HHS ID (Optional)
o 3c: Inpatient pediatric beds (Required February 2, 2022)
o 4c: Pediatric inpatient bed Occupancy (Required February 2, 2022)
o 5c: Pediatric ICU beds (Required February 2, 2022)
o 6c: Pediatric ICU occupancy (Required February 2, 2022)
o 12c: Hospitalized ICU pediatric laboratory-confirmed COVID-19 patients (Required February 2, 2022)
o 18c: Previous day’s pediatric admissions with laboratory-confirmed COVID-19 breakdown by age group (Required February 2,
2022)
o 40c: Therapeutic D on hand (Required January 19, 2022)
o 40d: Therapeutic D administered (Required January 19, 2022)
• Existing Data Elements Made Required:
o 33: Hospitalized patients with laboratory-confirmed influenza virus infection
o 34: Previous day’s influenza admissions with laboratory-confirmed influenza virus infection
o 35: Total hospitalized ICU patients with laboratory confirmed influenza virus infection
• Data Elements Changed to a Weekly Cadence:
o 24: Critical staffing shortage anticipated within a week (Y/N)
• Data Elements Made Inactive for the Federal Data Collection
5
:
o 2a: All Hospital Beds
o 2b: All Adult Hospital Beds
o 7: Total Mechanical Ventilators
o 8: Ventilators in Use
o 14: ED Overflow
o 15: ED Overflow and Ventilated
5
Note: Data elements are referred to in short-hand for enhanced readability. Full descriptions of previous data elements will be available in archived versions of
the hospital reporting guidance available on the Templates and Technical Materials page.
37
o 16: Previous Day’s COVID-19 Deaths
o 21: Previous Day’s Remdesivir Used
o 22: Current Inventory Remdesivir
o 23: Critical Staffing Shortage Today
o 25: Additional Details, Staffing
o 26: PPE Management at Facility or Centrally
o 27a: Days On Hand, Ventilator Supplies
o 28a: Eaches, N95 Respirators
o 28b: Eaches, Other Respirators
o 28c: Eaches, Surgical and Procedural Masks
o 28d: Eaches, Eye Protection
o 28e: Eaches, Single Use Gowns
o 28f: Eaches, Launderable Gowns
o 28g: Eaches, Exam Gloves
o 29a: Ability to Obtain, Ventilator Supplies
o 29b: Ability to Obtain, Ventilator Medications
o 29c: Ability to Obtain, N95 Respirators
o 29d: Ability to Obtain, Other Respirators
o 29e: Ability to Obtain, Surgical and Procedural Masks
o 29f: Ability to Obtain, Eye Protection
o 29g: Ability to Obtain, Single Use Gowns
o 29h: Ability to Obtain, Exam Gloves
o 29i: Ability to Maintain, Supply of Launderable Gowns
o 30a: Ability to Maintain, Ventilator Supplies
o 30b: Ability to Maintain, Ventilator Medications
o 30d: Ability to Maintain, Other Respirators
o 30i: Ability to Maintain, Nasal Pharyngeal Swabs
o 30j: Ability to Maintain, Nasal Swabs
o 30k, Ability to Maintain, Viral Transport Media
o 31a: Re-use Gowns
o 31b: Re-use PAPRs
o 31c: Re-use N95 Respirators
o 32: Additional Details, Supplies
o 36: Hospitalized Co-infection Influenza and COVID-19

38
o 37: Previous Day’s Influenza Deaths
o 38: Previous Day’s Deaths Co-infected with Influenza and COVID-19
o 39c: Therapeutic B Inventory On Hand
o 39d: Therapeutic B Courses Administered
• Data Elements with Clarified Definitions:
o 3a, 3b, 5a, 5b: Clarified definitions of staffed beds to beds that are currently set-up, staffed and able to be used for a patient within
the reporting period.
o 5a,5b: Added clarification on ICU bed location.
o 9a-18c: Changed from “Confirmed Positive” to “Laboratory-Confirmed”, included the definition of laboratory-confirmed.
o 11: Added definition of mechanical ventilators.
o 13: Removed note for COVID-19 isolation precautions.
o 18a, 18b: Added age and inpatient bed clarifications for pediatric patients.
o 20: Added definitions of “Confirmed Suspected” and “Laboratory-Confirmed”, included definitions. Minor non-substantive edits
to wording.
o 24: Removed staffing types.
o 27: Removed duplicative supply list from description.
o 30: Added question response options (Y, N, N/A).
o Influenza field overview: Moved to appendix D.
o 39b, 40b, 40d: Clarified the preferred “week” for reporting is Wednesday-Tuesday
o Therapeutic placeholder field overview: Minor non-substantive edits to wording.
o Vaccination field overview: Noted CMS rule CMS-1752-F and CMS-1762-F which requires hospital worker vaccination rates to
be reported on a regular basis into the National Healthcare Safety Network (NHSN) as a quality measure beginning on October 1,
2021. Clarified that vaccination data elements remain optional and do not meet the requirements of the CMS rule. Condensed
other description information.
o 41: Removed note on vaccine allocations.
o All subset fields: Clarified subset relationships, which can also be found in visual form with Appendix E.
Laboratory Data Element Changes
While the laboratory data elements themselves, as well as the guidance on how to report have not changed, the latest guidance clarifies that all
states and territories are now reporting line-level de-identified data electronically and hospitals should stop reporting directly to HHS unless
circumstances change.
39
Since hospitals no longer need to report the information directly and all details and frequently asked questions are readily available on the CDC
website for how to report laboratory data, all of the text describing laboratory data elements was removed from the guidance. Hospitals are still
required to report the information to their state through existing public health mechanisms.
Narrative and FAQ Changes
• The narrative and FAQs were streamlined and reorganized for clarity.
• Information was added throughout the document regarding data driving principles, purpose, and utility.
• Reporting information was reformatted for clarity.
• Multiple appendices, including clarifying information, were added for enhanced user friendliness.
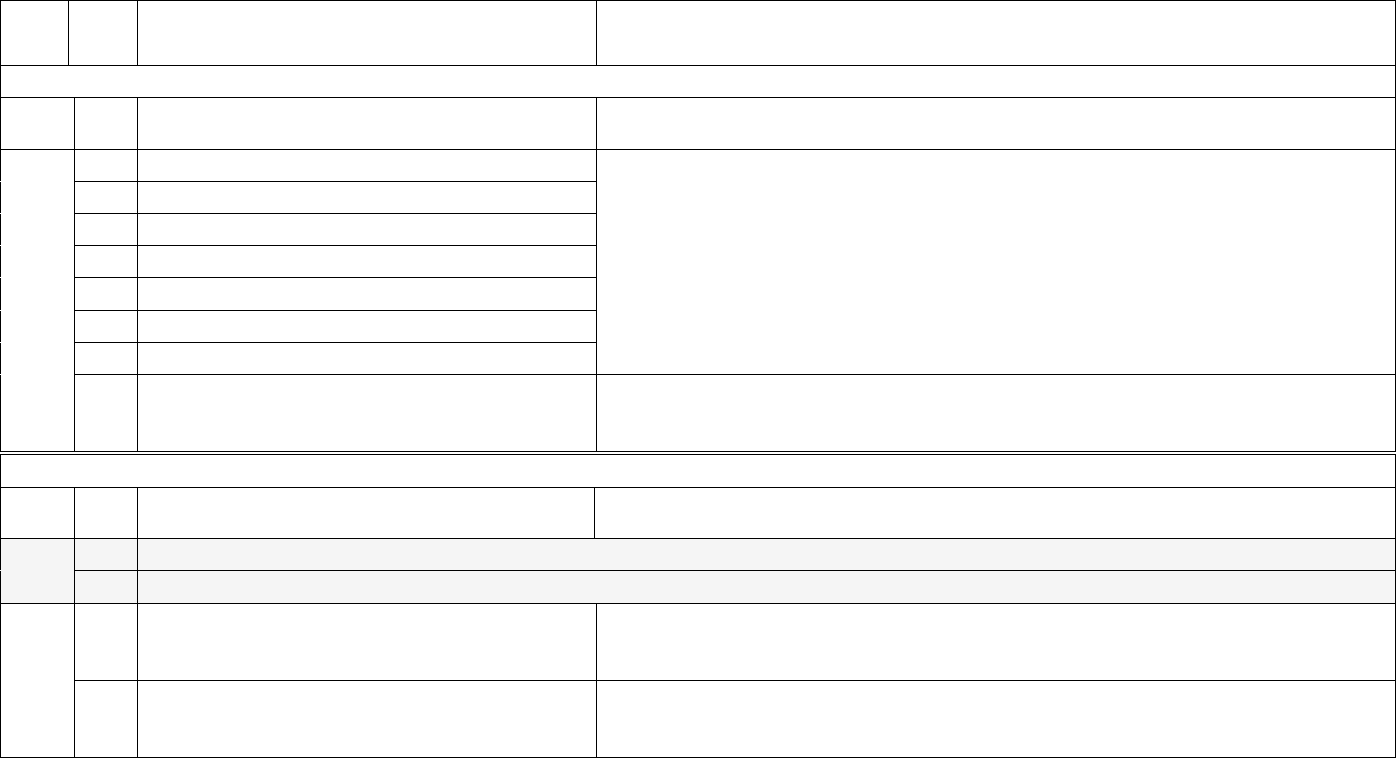
40
Appendix B: Data Element Purpose
The below table describes how each data element is used to inform the Federal COVID-19 response.
ID
Sub
ID
Information Needed
Purpose
Metadata
ID
Sub
ID
Information Needed
Purpose
1
a.
Hospital Name
Metadata ensures data can be identified and matched with the appropriate facility.
Logic is incorporated into NHSN (and should be incorporated into other systems) so
facilities do not need to answer metadata questions unless there are changes.
1
b.
CCN
1
c.
NHSN Org ID
1
d.
State
1
e.
County
1
f.
ZIP
1
g.
TeleTracking ID
1
h.
HHS ID
Serving as an additional metadata component, HHS ID is a unique facility-level
identifier which is more granular than CCN. Not having the HHS ID in the dataset has
caused some data submissions to be mismatched.
Capacity, Occupancy, Hospitalizations, and Admissions
ID
Sub
ID
Information Needed
Purpose
2
a.
This field has been made inactive for the federal data collection. (all hospital beds)
2
b.
This field has been made inactive for the federal data collection. (all adult hospital beds)
3
a.
All hospital inpatient beds
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. All hospital inpatient beds are required for
calculations such as the number of admissions per 100 beds.
3
b.
Adult hospital inpatient beds (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. Adult hospital inpatient beds are required for
analysis of number of adult and pediatric inpatient beds available.
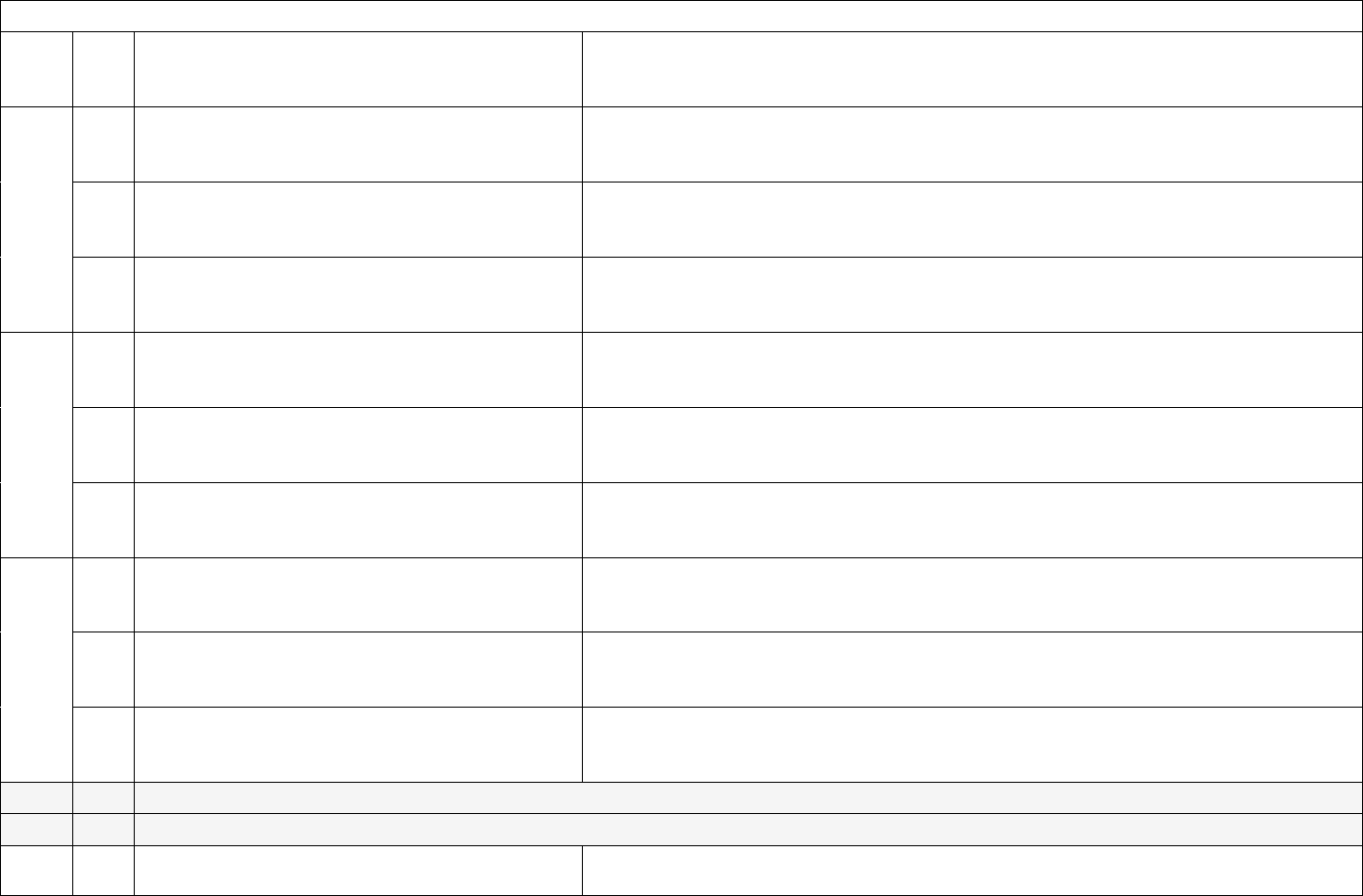
41
Capacity, Occupancy, Hospitalizations, and Admissions
3
c.
Inpatient pediatric beds (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. Explicit fields on inpatient pediatric beds will aid
to more fully understand pediatric capacity.
4
a.
All hospital inpatient bed occupancy
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for analysis of national
inpatient occupancy.
4
b.
Adult hospital inpatient bed occupancy (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for analysis of national adult
inpatient occupancy.
4
c.
Pediatric hospital inpatient bed occupancy
(Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. Explicit fields on inpatient pediatric bed
occupancy will help to more fully understand pediatric capacity.
5
a.
ICU beds (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for analysis of national ICU
bed availability.
5
b.
Adult ICU beds (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for analysis of national adult
ICU bed availability.
5
c.
Pediatric ICU beds (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for analysis of national
pediatric ICU bed availability.
6
a.
ICU bed occupancy (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for understanding national ICU
bed occupancy.
6
b.
Adult ICU bed occupancy (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for understanding national adult
ICU bed occupancy.
6
c.
Pediatric ICU bed occupancy (Subset)
The capacity and occupancy fields are used to inform Federal understanding of areas
experiencing surges in hospital stress. This field is used for understanding national
pediatric ICU bed occupancy.
7
This field has been made inactive for the federal data collection. (Total mechanical ventilators)
8
This field has been made inactive for the federal data collection. (Mechanical Ventilators in Use)
9
a.
Total hospitalized adult suspected or laboratory-
confirmed COVID-19 patients
This field could be helpful in the event of testing delays and/or disruptions.
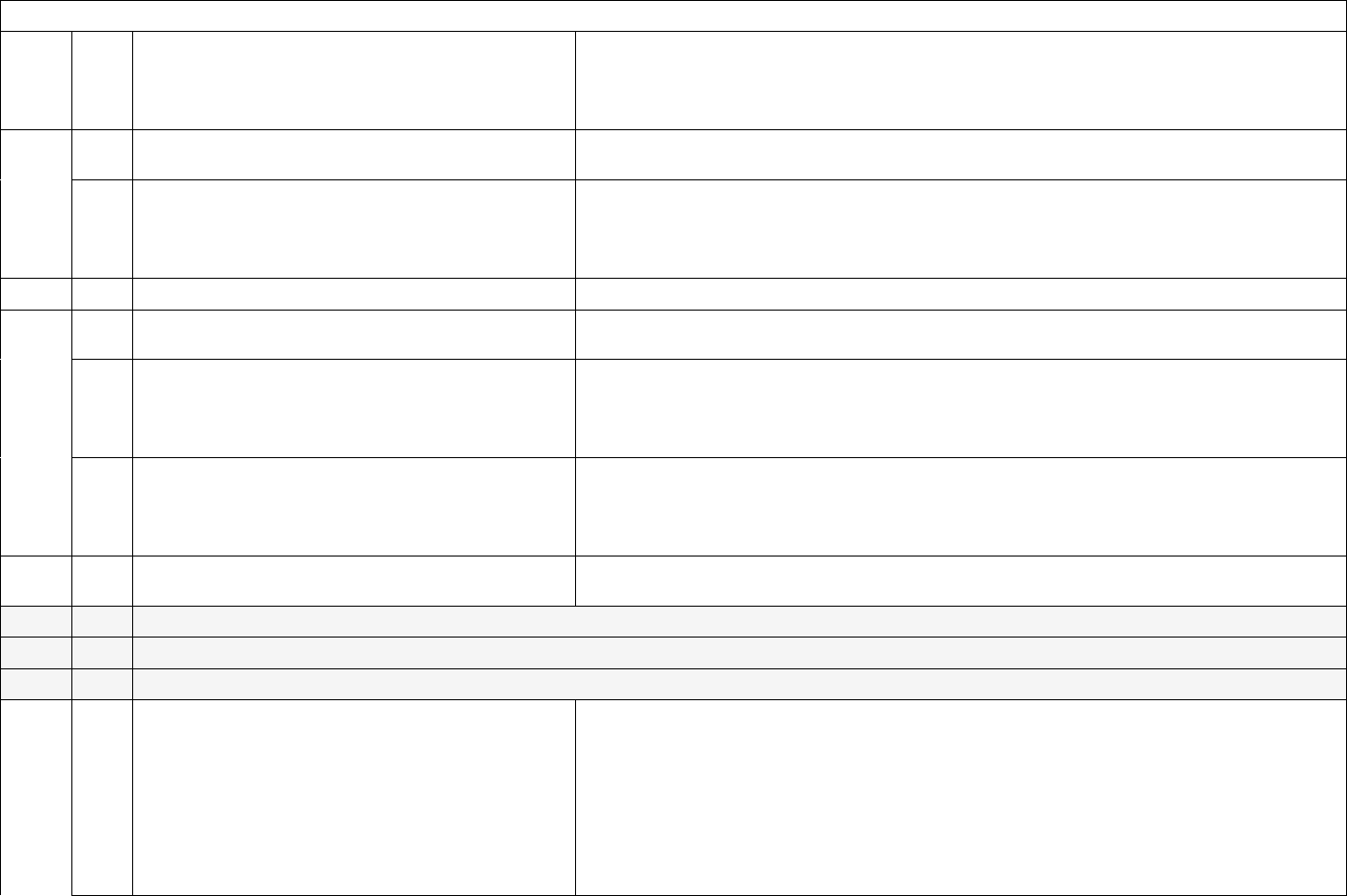
42
Capacity, Occupancy, Hospitalizations, and Admissions
9
b.
Hospitalized adult laboratory-confirmed COVID-
19 patients
Total adult patients currently hospitalized with laboratory-confirmed COVID-19 is a
key surveillance indicator for understanding severe COVID-19 epidemiology in the
U.S. and which areas are experiencing higher burden. This field is also used for various
public-facing visualizations and 7-day rolling averages.
10
a.
Hospitalized pediatric suspected or laboratory-
confirmed COVID-19 patients
This field could be helpful in the event of testing delays and/or disruptions
10
b.
Hospitalized pediatric laboratory-confirmed
COVID-19 patients
Total patients currently hospitalized in a pediatric inpatient bed with laboratory-
confirmed COVID-19 is a key surveillance indicator for understanding severe COVID-
19 epidemiology among children and adolescents in the U.S. and which areas are
experiencing higher burden.
11
Hospitalized and ventilated COVID-19 patients
This measure serves as an indication of COVID-19 severity.
12
a.
Total ICU adult suspected or laboratory-
confirmed COVID-19 patients
This field could be helpful in the event of testing delays and/or disruptions.
12
b.
Hospitalized ICU adult laboratory-confirmed
COVID-19 patients
Total adult patients currently in an ICU bed with laboratory-confirmed COVID-19 is a
key surveillance indicator for understanding the most severe COVID-19 cases in the
U.S. and which areas are experiencing higher burden. This is also an important
indicator for monitoring hospital stress of COVID-19.
12
c.
Hospitalized ICU pediatric laboratory-confirmed
COVID-19 patients
This measure serves as a key surveillance indicator for understanding the most severe
pediatric COVID-19 cases, and which areas are experiencing higher burden related to
pediatric cases. This is also an important indicator for monitoring hospital stress of
COVID-19, especially for pediatric capabilities.
13
Hospital Onset
This field could be helpful to identify the prevalence of hospital acquired infections of
COVID-19.
14
This field has been made inactive for the federal data collection. (ED/Overflow)
15
This field has been made inactive for the federal data collection. (ED/Overflow and Ventilated)
16
This field has been made inactive for the federal data collection. (Previous day’s COVID-19 Deaths)
17
a.
Previous day’s adult admissions with laboratory-
confirmed COVID-19 and breakdown by age
bracket:
• 18-19
• 20-29
• 30-39
• 40-49
Previous day admissions of patients with laboratory-confirmed COVID-19 is the
primary surveillance indicator used to monitor the epidemiology of severe COVID-19
and trends by age group in the U.S. These fields are monitored closely on a daily basis
and used to inform federal understanding of changes in trends, and these fields are often
combined with other data sources to identify areas of concern in the U.S.
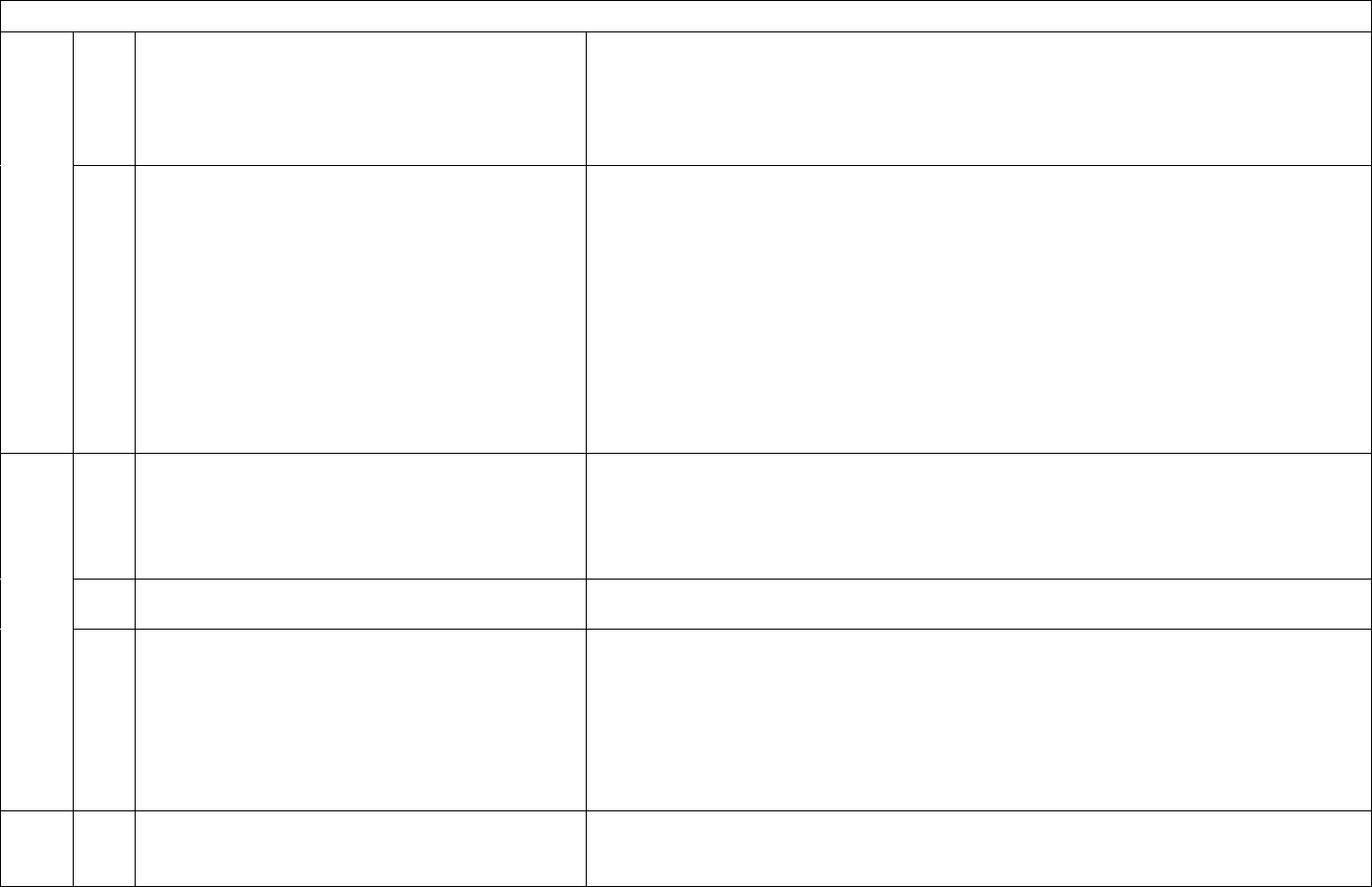
43
Capacity, Occupancy, Hospitalizations, and Admissions
• 50-59
• 60-69
• 70-79
• 80+
• Unknown
17
b.
Previous day’s adult admissions with suspected
COVID-19 and breakdown by age bracket:
• 18-19
• 20-29
• 30-39
• 40-49
• 50-59
• 60-69
• 70-79
• 80+
• Unknown
This field could be helpful in the event of testing delays and/or disruptions.
18
a.
Previous day’s pediatric admissions with
laboratory-confirmed COVID-19:
Previous day pediatric admissions of patients with laboratory-confirmed COVID-19 is a
primary surveillance indicator used to monitor the epidemiology of severe COVID-19
in children and adolescents. This fields is monitored closely on a daily basis and used to
inform federal understanding of changes in trends and how pediatric admissions
compare to adult, and to identify areas of concern in the U.S.
18
b.
Previous day’s pediatric admissions with
suspected COVID-19
This field could be helpful in the event of testing delays and/or disruptions.
18
c.
Previous day’s pediatric admissions with
laboratory-confirmed COVID-19; stratification
by age group:
• 0-4
• 5-11
• 12-17
• Unknown
Previous day pediatric admissions of patients with laboratory-confirmed COVID-19 is a
primary surveillance indicator used to monitor the epidemiology of severe COVID-19
in children and adolescents. Additional age information can help to better understand
epidemiologic trends. This fields will be monitored closely on a daily basis and used to
inform federal understanding of changes in trends and how pediatric admissions
compare to adult, and to identify areas of concern in the U.S.
19
Previous day’s ED Visits
Previous day total ED visits, in conjunction with COVID-19 ED visits, is used to
monitor the epidemiology of COVID-19 by percentage of ED visits for COVID-19 and
trends by region in the U.S. These fields are used by the National Syndromic
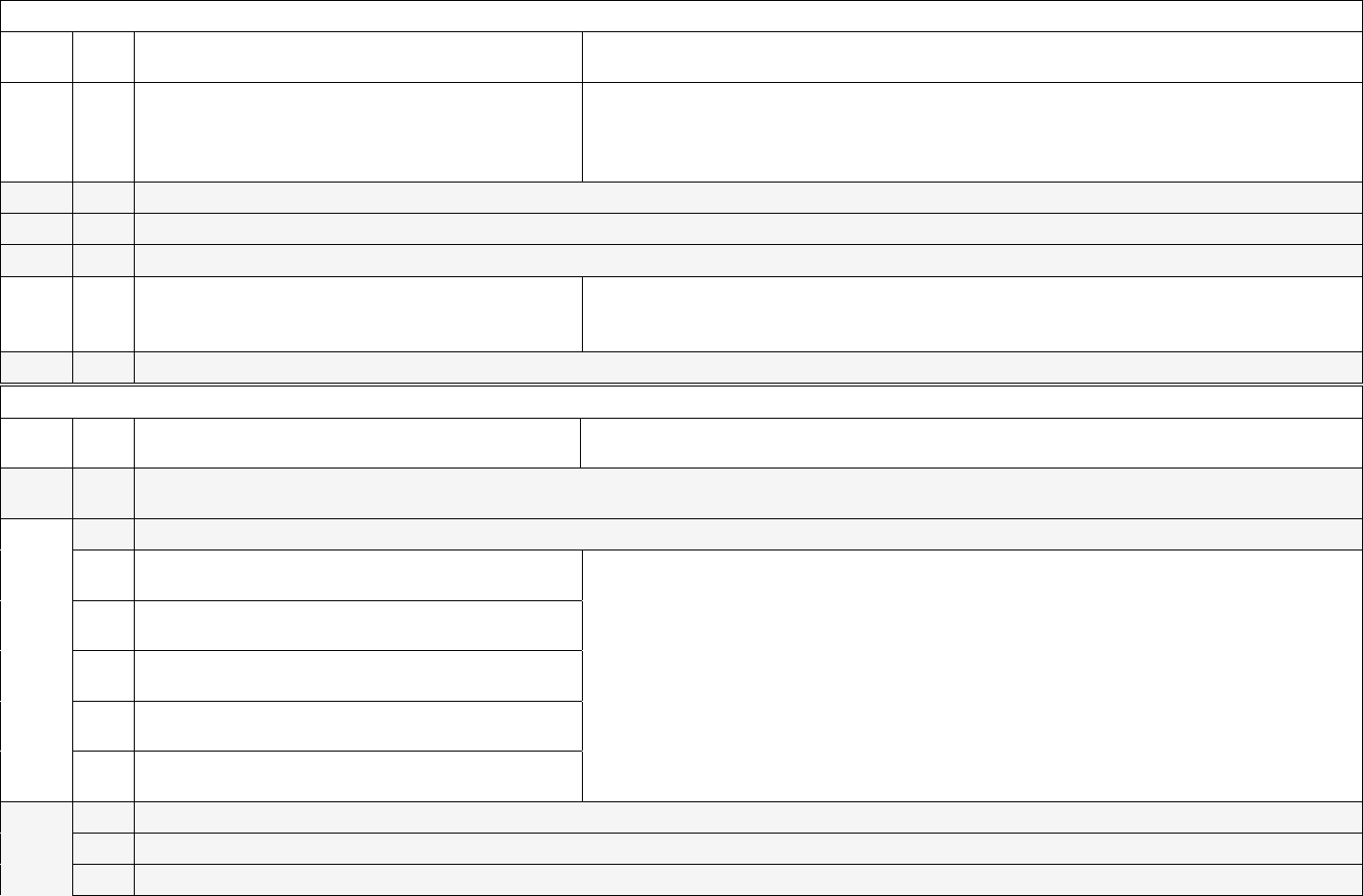
44
Capacity, Occupancy, Hospitalizations, and Admissions
Surveillance Program (NSSP) to fill in COVID-19 ED data for the 30% of U.S.
hospitals not covered by NSSP.
20
Previous day’s total COVID-19- related ED
visits (Subset)
Previous day total COVID-19 ED visits, in conjunction with total ED visits, is used to
monitor the epidemiology of COVID-19 and trends by region in the U.S. These fields
are used by the National Syndromic Surveillance Program (NSSP) to fill in COVID-19
ED data for the 30% of U.S. hospitals not covered by NSSP.
21
This field has been made inactive for the federal data collection. (Previous day’s remdesivir used)
22
This field has been made inactive for the federal data collection. (Current inventory of remdesivir)
23
This field has been made inactive for the federal data collection. (Critical staffing shortage today (Y/N)
24
Critical staffing shortage anticipated within a
week (Y/N)
This field can help to glean information on critical staffing shortages, helping to inform
policy decisions and other potential staffing solutions. This question can also help to
inform decisions related to requests for personnel.
25
This field has been made inactive for the federal data collection. (Staffing shortage details)
Supplies
ID
Sub
ID
Information Needed
Purpose
26
This field has been made inactive for the federal data collection. (Are your PPE supply items managed (purchased, allocated, and/or
stored) at the facility level or centrally)
27
a.
This field has been made inactive for the federal data collection. (On hand Ventilator Supplies)
27
b.
On hand supply duration in days: N95
respirators
Allows HHS to assess current PPE resiliency in the event of a supply chain disruption,
for a single hospital or for hospitals overall in a local area, state, or nationwide.
27
c.
On hand supply duration in days: Surgical and
procedure masks
Allows HHS to assess current PPE resiliency in the event of a supply chain disruption,
for a single hospital or for hospitals overall in a local area, state, or nationwide.
27
d.
On hand supply duration in days: Eye protection
including face shields and goggles
Allows HHS to assess current PPE resiliency in the event of a supply chain disruption,
for a single hospital or for hospitals overall in a local area, state, or nationwide.
27
e.
On hand supply duration in days: Single-use
gowns
Allows HHS to assess current PPE resiliency in the event of a supply chain disruption,
for a single hospital or for hospitals overall in a local area, state, or nationwide.
27
f.
On hand supply duration in days: Exam gloves
(sterile and non-sterile)
Allows HHS to assess current PPE resiliency in the event of a supply chain disruption,
for a single hospital or for hospitals overall in a local area, state, or nationwide.
28
a.
This field has been made inactive for the federal data collection. (Eaches, n95 respirators)
28
b.
This field has been made inactive for the federal data collection. (Eaches, other respirators)
28
c.
This field has been made inactive for the federal data collection. (Eaches, surgical & procedural masks)

45
Supplies
28
d.
This field has been made inactive for the federal data collection. (Eaches, eye protection)
28
e.
This field has been made inactive for the federal data collection. (Eaches, single-use gowns)
28
f.
This field has been made inactive for the federal data collection. (Eaches, launderable gowns)
28
g.
This field has been made inactive for the federal data collection. (Eaches, exam gloves)
29
a.
This field has been made inactive for the federal data collection. (Able to obtain, ventilator supplies)
29
b.
This field has been made inactive for the federal data collection. (Able to obtain, ventilator medications)
29
c.
This field has been made inactive for the federal data collection. (Able to obtain, n95 respirators)
29
d.
This field has been made inactive for the federal data collection. (Able to obtain, other respirators)
29
e.
This field has been made inactive for the federal data collection. (Able to obtain, surgical & procedural masks)
29
f.
This field has been made inactive for the federal data collection. (Able to obtain, eye protection)
29
g.
This field has been made inactive for the federal data collection. (Able to obtain, single-use gowns)
29
h.
This field has been made inactive for the federal data collection. (Able to obtain, exam gloves)
29
i.
This field has been made inactive for the federal data collection. (Able to maintain, launderable gowns)
30
a.
This field has been made inactive for the federal data collection. (Able to maintain, ventilator supplies)
30
b.
This field has been made inactive for the federal data collection. (Able to maintain, ventilator medications)
30
c.
Are you able to maintain at least a 3-day supply
of N95 respirators?
HHS uses hospitals’ self-assessment of the reliability of their PPE supply to identify
areas or patterns of unreliable supply that may warrant outreach and (if needed)
interventions to stabilize the supply chain.
30
d.
This field has been made inactive for the federal data collection. (Able to maintain, other respirators)
30
e.
Are you able to maintain at least a 3-day supply
of surgical and procedural masks?
HHS uses hospitals’ self-assessment of the reliability of their PPE supply to identify
areas or patterns of unreliable supply that may warrant outreach and (if needed)
interventions to stabilize the supply chain.
30
f.
Are you able to maintain at least a 3-day supply
of eye protection including face shields and
goggles?
HHS uses hospitals’ self-assessment of the reliability of their PPE supply to identify
areas or patterns of unreliable supply that may warrant outreach and (if needed)
interventions to stabilize the supply chain.
30
g.
Are you able to maintain at least a 3-day supply
of single-use gowns?
HHS uses hospitals’ self-assessment of the reliability of their PPE supply to identify
areas or patterns of unreliable supply that may warrant outreach and (if needed)
interventions to stabilize the supply chain.
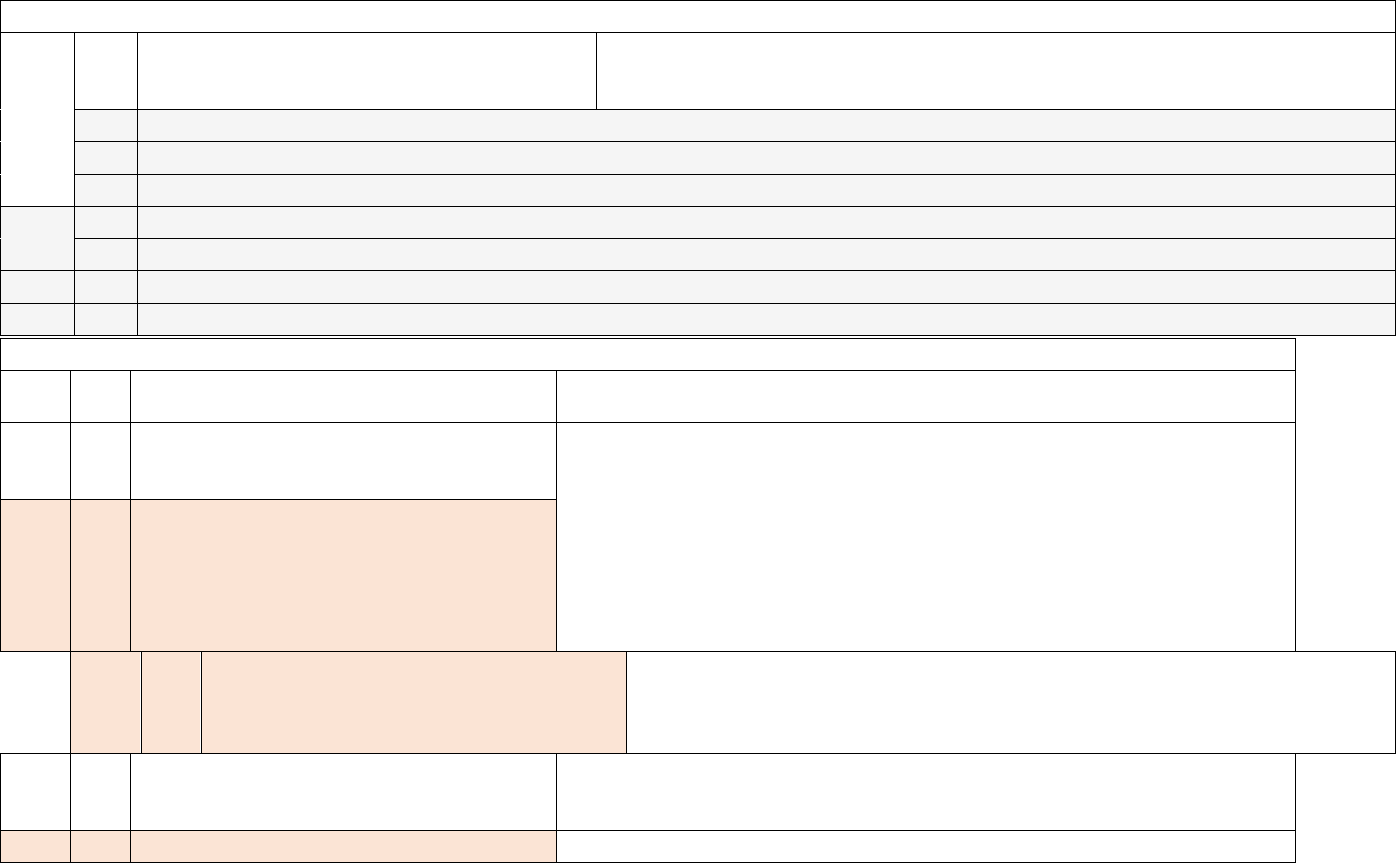
46
Supplies
30
h.
Are you able to maintain at least a 3-day supply
of exam gloves?
HHS uses hospitals’ self-assessment of the reliability of their PPE supply to identify
areas or patterns of unreliable supply that may warrant outreach and (if needed)
interventions to stabilize the supply chain.
30
i.
This field has been made inactive for the federal data collection. (Able to maintain, nasal pharyngeal swabs)
30
j.
This field has been made inactive for the federal data collection. (Able to maintain, nasal swabs)
30
k.
This field has been made inactive for the federal data collection. (Able to maintain, viral transport media)
31
a.
This field has been made inactive for the federal data collection. (Reuse gowns)
31
b.
This field has been made inactive for the federal data collection. (Reuse PAPRS)
31
c.
This field has been made inactive for the federal data collection. (Reuse n95 respirators)
32
This field has been made inactive for the federal data collection. (Critical issues)
Influenza
ID
Sub
ID
Information Needed
Purpose
33
Total hospitalized patients with laboratory-
confirmed influenza virus infection
Seasonal influenza can result in substantial burden on hospitals. These data
elements fill a critical gap in the national influenza surveillance system by
providing hospitalization data from all states and territories. These data will be
used to improve situational awareness of severe respiratory illness, make
forecasts and model influenza impact, help direct resources to address the
potential increased impact of flu and COVID-19 co-circulation and inform
guidance and recommendations for public health professionals, clinicians, and
the general public. Understanding influenza hospitalizations and admissions can
also help to understand potential strains on the PPE supply chain.
a.
[NEW]
Hospitalized adult patients with laboratory-
confirmed influenza virus infection
b.
[NEW]
Hospitalized pediatric patients with
laboratory-confirmed influenza virus
infection
34
Previous day’s influenza admissions
(laboratory- confirmed influenza virus
infection)
a.
[NEW]
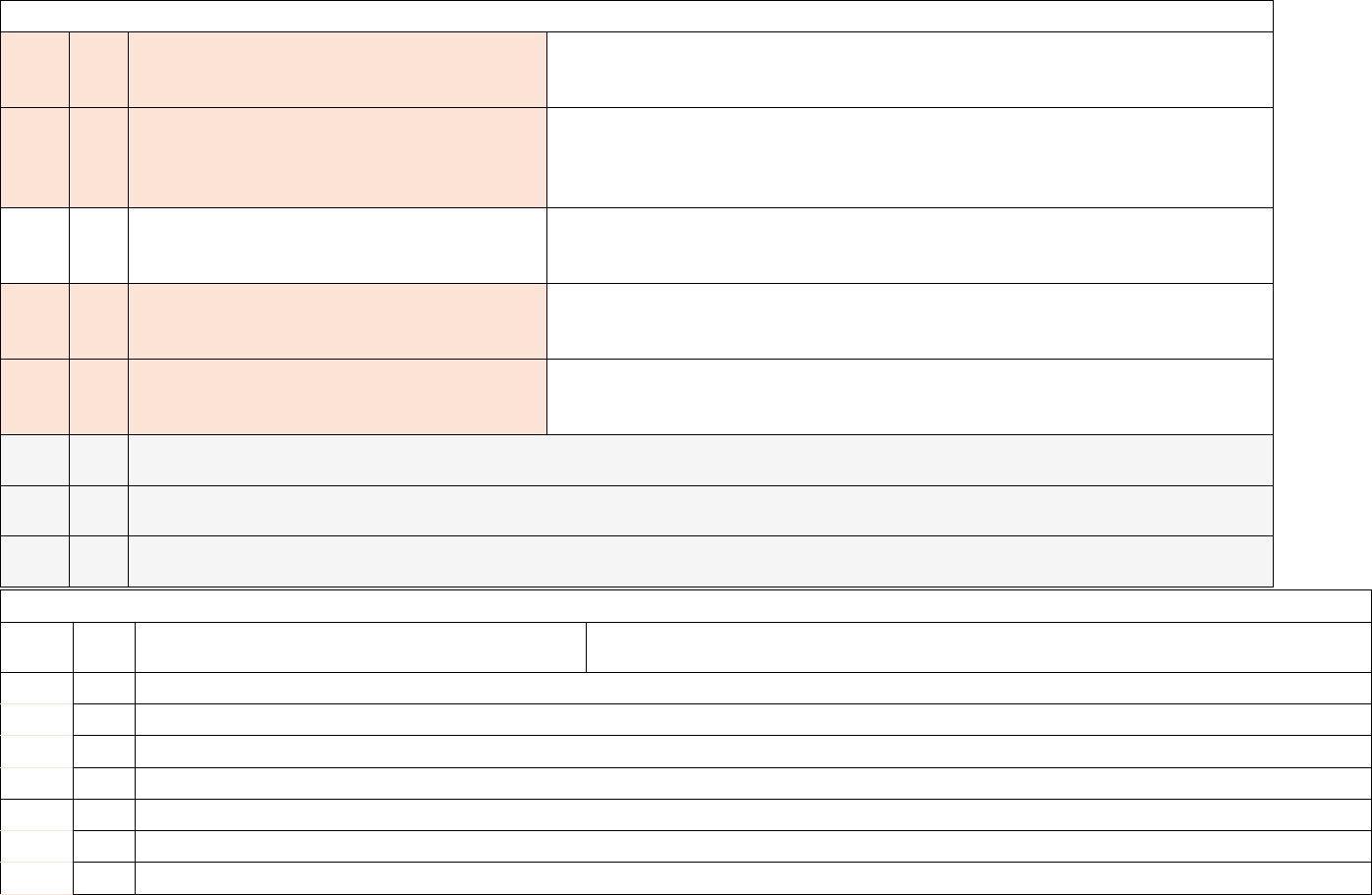
47
Influenza
Previous day’s adult admissions with
laboratory-confirmed influenza virus
infection
b.
[NEW]
Previous day’s pediatric admissions with
laboratory-confirmed influenza virus
infection
35
Total hospitalized ICU patients with
laboratory-confirmed influenza virus
infection
a.
[NEW]
Hospitalized ICU adult laboratory-confirmed
influenza patients
b.
[NEW]
Hospitalized ICU pediatric laboratory-
confirmed influenza patients
36
This field has been made inactive for the federal data collection. (Total hospitalized patients co- infected with both laboratory-
confirmed COVID- 19 and laboratory-confirmed influenza virus infection)
37
This field has been made inactive for the federal data collection. (Previous day’s influenza deaths (laboratory-confirmed
influenza virus infection)
38
This field has been made inactive for the federal data collection. (Previous day’s deaths for patients co-infected with both
COVID-19 and laboratory- confirmed influenza virus)
Therapeutic
ID
Sub
ID
Information Needed
Purpose
39
a.
This field was moved to HPOP on November 2, 2022. (Therapeutic A Courses on Hand)
39
b.
This field was moved to HPOP on November 2, 2022. (Therapeutic A Courses Administered in Last Week )
39
c.
This field has been made inactive for the federal data collection. (Therapeutic B on hand)
39
d.
This field has been made inactive for the federal data collection. (Therapeutic B courses)
40
a.
This field wasmoved to HPOP on November 2, 2022. (Therapeutic C Courses on Hand)
40
b.
This field was moved to HPOP on November 2, 2022. (Therapeutic C Courses Administered in Last Week)
40
c.
This field was moved to HPOP on November 2, 2022. (Therapeutic D Courses on Hand)
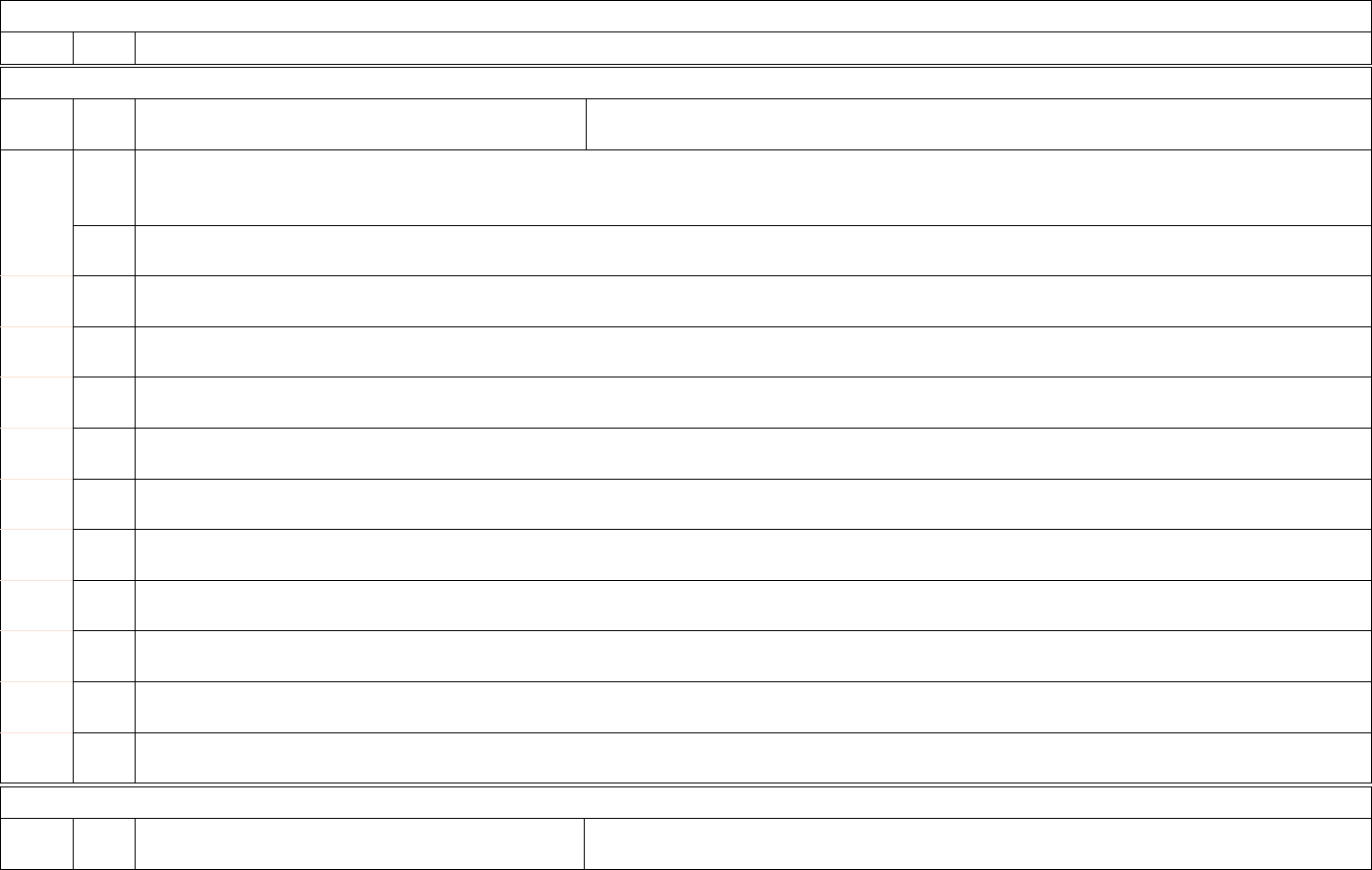
48
Therapeutic
40
d.
This field was moved to HPOP on November 2, 2022. (Therapeutic D Courses Administered in Last Week)
Therapeutic Placeholders
ID
Sub
ID
Information Needed
Purpose
40
e.
As of August 10, 2022, therapeutic placeholders are inactive due to all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic E Courses on Hand)
40
f.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic E Courses Administered in Last Week)
40
g.
As of August 10, 2022, therapeutic placeholders are inactive due to the incoming change of all therapeutic reporting being moved into
HPOP on November 2, 2022. (Placeholder, Therapeutic F Courses on Hand)
40
h.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic F Courses Administered in Last Week)
40
i.
As of this August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November
2, 2022. (Placeholder, Therapeutic G Courses on Hand)
40
j.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic G Courses Administered in Last Week)
40
k.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic H Courses on Hand)
40
l.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic H Courses Administered in Last Week)
40
m.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic I Courses on Hand)
40
n.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic I Courses Administered in Last Week)
40
o.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic J Courses on Hand)
40
p.
As of August 10, 2022, therapeutic placeholders are inactive due to of all therapeutic reporting being moved into HPOP on November 2,
2022. (Placeholder, Therapeutic J Courses Administered in Last Week)
Healthcare Worker Vaccination
ID
Sub
ID
Information Needed
Purpose
49
Healthcare Worker Vaccination
41
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Previous week’s COVID-19 vaccination doses administered to healthcare personnel by your facility (Regardless of series or single-dose
vaccine))
42
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Current healthcare personnel who have not yet received any COVID-19 vaccination doses)
43
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Current healthcare personnel who have received the first dose in a multi-series of COVID-19 vaccination doses)
44
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Current healthcare personnel who have received a completed series of a COVID-19 vaccination or a single-dose vaccination)
45
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance. (Total
number of current healthcare personnel)
46
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Previous week’s number of patients and other non- healthcare personnel who received the first dose in a multi-series of COVID-19
vaccination doses)
47
This field has been made inactive for the federal data collection. Hospitals no longer need to report these data elements to the federal
government through the Unified Hospital Data Surveillance System. Please ensure complete reporting to NHSN per CMS guidance.
(Previous week’s number of patients who received the final dose in a series of COVID-19 vaccination doses or the single- dose vaccine by
your facility)
Respiratory Syncytial Virus (RSV)
ID
Sub
ID
Information Needed
Purpose
48
a.
[NEW]
Hospitalized adult patients with laboratory-
confirmed RSV infection
RSV can result in substantial burden on hospitals. These data elements fill a critical gap
in the national RSV surveillance system by providing hospitalization data from all
states and territories. These data will be used to improve situational awareness of severe
respiratory illness, make forecasts and model RSV impact, help direct resources to
b.
[NEW]
50
Respiratory Syncytial Virus (RSV)
Hospitalized pediatric patients with laboratory-
confirmed RSV infection
address the potential increased impact of RSV, flu, and COVID-19 co-circulation and
inform guidance and recommendations for public health professionals, clinicians, and
the general public. Understanding RSV hospitalizations and admissions can also help
to understand potential strains on the PPE supply chain.
49
a.
[NEW]
Previous day’s adult admissions with laboratory-
confirmed RSV infection
b.
[NEW]
Previous day’s pediatric admissions with
laboratory-confirmed RSV infection
50
a.
[NEW]
Hospitalized ICU adult laboratory-confirmed
RSV patients
b.
[NEW]
Hospitalized ICU pediatric laboratory-confirmed
RSV patients
51
Appendix C: Required and Optional Reporting Elements
The below table is intended to provide a quick reference of current required and optional data elements. The information is the same as the above
table in the “Data Elements” section, however, instead of being grouped by numerical value and field type, the data elements are grouped by
whether they are required, optional, turned off, or placeholders.
ID
Sub ID
Information Needed
Daily Required Data Elements, to be reported once weekly (by Tuesday 1159 pm local time, for all days in previous week, Sunday
through Saturday)
(All facilities are encouraged to back-date information from weekends and holidays by the following Tuesday)
ID
Sub ID
Information Needed
1
a.
Hospital Name
1
b.
Hospital CCN
c.
NHSN Org ID
1
d.
State
1
e.
County
1
f.
ZIP
3
a.
All hospital inpatient beds
3
b.
All adult inpatient beds
3
c.
All pediatric inpatient beds
4
a.
All hospital inpatient occupancy
4
b.
All adult inpatient occupancy
4
c.
All pediatric inpatient occupancy
5
a.
All ICU beds
5
b.
Adult ICU beds
5
c.
Pediatric ICU beds
6
a.
All ICU bed occupancy
6
b.
Adult ICU occupancy
52
ID
Sub ID
Information Needed
6
c.
Pediatric ICU occupancy
9
b.
Hospitalized adult laboratory-confirmed COVID-19 patients
10
b.
Hospitalized pediatric laboratory-confirmed COVID-19 patients
12
b.
Hospitalized ICU adult laboratory-confirmed COVID-19 patients
12
c.
Hospitalized ICU pediatric laboratory-confirmed COVID-19 patients
17
a. (includes age
ranges)
Previous day’s adult admissions with laboratory-confirmed COVID-19 and breakdown by age
18
a.
Previous day’s pediatric admissions with laboratory-confirmed COVID-19
18
c. (includes age
ranges)
Previous day’s pediatric admissions with laboratory-confirmed COVID-19 by age
33
Total hospitalized patients with laboratory-confirmed influenza virus infection
34
Previous day’s influenza virus infection admissions (laboratory-confirmed influenza virus infection)
35
Total hospitalized ICU patients with laboratory-confirmed influenza virus infection
Weekly Required Data Elements, to be reported once weekly on Tuesdays by 1159pm local time, for Wednesday collection date in
previous week
ID
Sub ID
Information Needed
27
b.
On hand supply (DURATION in days) n95 respirators
27
c.
On hand supply (DURATION in days) surgical and procedure masks
27
d.
On hand supply (DURATION in days) eye protection including face shields and goggles
27
e.
On hand supply (DURATION in days) single use gowns
27
f.
On hand supply (DURATION in days) exam gloves (sterile and non-sterile)
30
c.
Are you able to MAINTAIN at least a 3-day supply of these items (y/n/n/a)? N95 respirators
30
e.
Are you able to MAINTAIN at least a 3-day supply of these items (y/n/n/a)? Surgical and procedure masks
30
f.
Are you able to MAINTAIN at least a 3-day supply of these items (y/n/n/a)? Eye protection including face
shields and goggles
30
g.
Are you able to MAINTAIN at least a 3-day supply of these items (y/n/n/a)? Single use gowns
53
Weekly Required Data Elements, to be reported once weekly on Tuesdays by 1159pm local time, for Wednesday collection date in
previous week
30
h.
Are you able to MAINTAIN at least a 3-day supply of these items (y/n/n/a)? Exam gloves
39
a.
Required until November 2, 2022, then moved to HPOP. Therapeutic A courses on hand
b.
Required until November 2, 2022, then moved to HPOP. Therapeutic A courses administered in the last week
40
a.
Required until November 2, 2022, then moved to HPOP. Therapeutic C courses on hand
b.
Required until November 2, 2022, then moved to HPOP. Therapeutic C courses administered in the last week
c.
Required until November 2, 2022, then moved to HPOP. Therapeutic D courses on hand
d.
Required until November 2, 2022, then moved to HPOP. Therapeutic D courses administered in the last week
Daily Optional Data Elements
(All facilities are encouraged to back-date information from weekends and holidays by the following Tuesday)
ID
Sub ID
Information Needed
1
g.
TeleTracking ID
h.
HHS ID
9
a.
Total hospitalized adult suspected or laboratory-confirmed COVID-19 patients
10
a.
Total hospitalized pediatric suspected or laboratory-confirmed COVID-19 patients
11
Hospitalized and ventilated COVID-19 patients
12
a.
Total ICU adult suspected or laboratory- confirmed COVID-19 patients
13
Hospital onset
17
b.
(includes age
ranges)
Previous day’s adult admissions with suspected COVID-19 and breakdown by age
18
b.
Previous day’s pediatric admissions with suspected COVID-19
19
Previous day’s Emergency Department (ED) Visits
54
Daily Optional Data Elements
(All facilities are encouraged to back-date information from weekends and holidays by the following Tuesday)
20
Previous day’s total COVID-19- related ED visits
33
[NEW]
a.
Hospitalized adult patients with laboratory-confirmed influenza virus infection
[NEW]
b.
Hospitalized pediatric patients with laboratory-confirmed influenza virus infection
34
[NEW]
a.
Previous day’s adult admissions with laboratory-confirmed influenza virus infection
[NEW]
b.
Previous day’s pediatric admissions with laboratory-confirmed influenza virus infection
35
[NEW]
a.
Hospitalized ICU adult laboratory-confirmed influenza patients
[NEW]
b.
Hospitalized ICU pediatric laboratory-confirmed influenza patients
48
[CHANGE]
a.
Hospitalized adult laboratory-confirmed RSV patients
[CHANGE]
b.
Hospitalized pediatric laboratory-confirmed RSV patients
49
[CHANGE]
a.
Previous day’s adult admissions with laboratory-confirmed RSV
[CHANGE]
b.
Previous day’s pediatric admissions with laboratory-confirmed RSV
50
[CHANGE]
a.
Hospitalized ICU adult laboratory-confirmed RSV patients
[CHANGE]
b.
Hospitalized ICU pediatric laboratory-confirmed RSV patients
Weekly Optional Data Elements
ID
Sub ID
Information Needed
24
Critical staffing shortage anticipated within a week (Y/N)

55
Appendix D: Additional Information by Field Type
NHSN Org ID
The NHSN OrgID (Organization Identification Number; also called NHSN Facility ID) is a facility’s unique numeric identifier in the NHSN
application and is intended to be assigned to the brick-and-mortar level of the facility, based on its physical location and address. Further, the
NHSN orgID is important as it will be the primary identifier for facilities after the transition of the COVID-19 hospital data to NHSN and serve as
the primary key for merging historical and new data for analytic purposes moving forward.
HHS ID
HHS IDs are specified and maintained for the purposes of providing granular facility level identifiers for the purposes of this COVID-19 Guidance
for Hospital Reporting. HHS IDs provide more granular information than CCN, as HHS ID references the individual facility level. HHS IDs for
each facility are published and listed in the “HHS IDs” file hosted on healthdata.gov.
NICU Exclusions & Inclusions
NICU and nursery beds are included in some fields in the collection while being excluded from others, unless they are designated for COVID-19
positive pediatric patients. This is based on several factors including making minimal changes to existing definitions, considering analysis of this
data collection combined with additional data sources, and reducing the number of new questions where feasible. The questions allow for
epidemiologic tracking of pediatric patients regardless of age or location in the hospital, COVID-19 burden analysis for specific areas of the
hospital, ability to more granularly track occupancy, and where needed potential to infer NICU occupancy. A diagram of capacity and occupancy
fields with additional notes on NICU fields is available in Appendix E.
NICU and nursery beds are included in:
• Overall capacity and occupancy measures (fields 3a, 4a, 5a, and 6a)
• Straight counts of pediatric patients who are hospitalized or admitted with COVID-19 regardless of age or location in the hospital (fields
10b, 18a, and 18c)
NICU and nursery beds are excluded in:
• New pediatric capacity and occupancy measures (fields 3c, 4c, 5c, and 6c)
• Measures of COVID-19 burden in pediatric ICUs (field 12c)

56
Additional Pediatric Reporting Clarifications
For facilities without beds designated specifically for adult or pediatric patients, it is ok to report pediatric capacity as zero up until the point when
there is a pediatric patient occupying a bed, in which case numbers for fields 3c, 4c, 5c, and 6c are asked to be reflective of hospitalized pediatric
patients.
Hospitalizations and Admissions
The number of new admissions and the total patients hospitalized should generally not be the same value.
• Confirmed COVID-19 admissions are the number of new patients who were admitted to an inpatient bed on the previous calendar day
with confirmed COVID-19. This is a measure of incidence, or new patients coming into the hospital.
• Total patients hospitalized with confirmed COVID-19 are the current number of patients with confirmed COVID-19 occupying an
inpatient bed. This is a measure of prevalence, or current patients occupying a hospital bed.
If the values are reported such that the number of patients currently hospitalized are incorrectly reported as the number of new admissions, this can
cause the new admissions rate for the facility, county, and state to appear overinflated. Accuracy of these fields is important, as they are included
in a number of reports, dashboards, and datasets that are widely used by the public and the U.S. government.
A scenario example is provided below to assist in determining how to enter the data for these questions:
• On 9/8/2021, facility A had 12 adult patients with confirmed COVID-19 occupying inpatient beds at the time of data entry. On the prior
day (9/7/2021), 3 new adult patients with confirmed COVID-19 were admitted to the facility.
o The facility should enter 12 for question #9b (12 total adult patients are hospitalized with confirmed COVID-19 on 9/8/2021).
o The facility should enter 3 for question #17b (3 new adult patients with confirmed COVID-19 were admitted on the prior day).
Laboratory-Confirmed COVID-19 Definition
Do NOT include the following as “laboratory confirmed COVID-19”:
• ±Positive SARS-CoV-2 antigen test and negative SARS-CoV-2 NAAT (PCR).
Laboratory-confirmed COVID-19 positive includes:
• Positive SARS-CoV-2 antigen test only [no other testing performed]
• Positive SARS-CoV-2 NAAT (PCR) only [no other testing performed]
• ±Any other combination of SARS-CoV-2 NAAT (PCR) and/or antigen test(s) with at least one positive test.

57
± Include patient with serial viral test results only when the additional tests were collected within two calendar days of initial SARS-CoV-2 viral
test. Day of specimen collection is equal to day 1. Otherwise, only select the initial test method for Test Type. Tests in which specimens are
collected more than 2 calendar days apart should be considered separate tests.
Note: Several hospitals have asked for clarification on how long someone who has met the conditions for laboratory-confirmed COVID-19
remains a COVID-19 patient. We recognize that some hospitals and STLT partners have made internal definitions that have been used since
reporting began. For some, a COVID-19 patient remains a COVID-19 patient for the duration of their stay, regardless of length of stay. For
others, a COVID-19 patient stops being a COVID-19 patient after two weeks. For the purposes of reporting, hospitals are asked to please
continue to use definitions that they have used for reporting to date. For new hospitals who are starting to report, please defer first to the COVID-
19 patient definition used by your hospital system, health care coalition, hospital association, and/or STLT partner. If a definition has not been
previously determined, a default definition we suggest is for individuals to be counted as COVID-19 patients until they are no longer symptomatic
and are removed from COVID-19 isolation precautions.
Laboratory-Confirmed Influenza Virus Infection Definition
Laboratory confirmation includes detection of influenza virus infection through molecular tests (e.g., polymerase chain reaction, nucleic acid
amplification), antigen detection tests, immunofluorescence tests, and virus culture. For hospital reporting, laboratory-confirmed influenza is
defined as Influenza A and B [this includes their subtypes and lineages (e.g., A(H1N1), A(H3N2), B/Victoria, B/Yamagata)]. Parainfluenza and
Haemophilus Influenza should not be reported. A positive result in the prior 14 days whether completed as an inpatient or outpatient can be used
as the laboratory confirmation.
[CHANGE] Laboratory-Confirmed Respiratory Syncytial Virus (RSV) Infection Definition
Laboratory confirmation includes detection of RSV infection through molecular tests (e.g., polymerase chain reaction, nucleic acid amplification),
antigen detection tests, immunofluorescence tests, and virus culture. A positive result in the prior 8 days whether completed as an inpatient or
outpatient can be used as the laboratory confirmation.
Psychiatric & Rehabilitation Hospital Reporting
As of August 10, 2022, per Secretary discretion, psychiatric and rehabilitation facilities must submit data federally only once on an annual basis
which will go from October to October. This may evolve based on the needs of the national response. All hospitals are asked to follow the
direction of their state and jurisdiction to ensure reporting meets STLT needs.
• As long as psychiatric & rehabilitation hospitals have reported once since October 2021, psychiatric & rehabilitation hospital federal
reporting requirements through October 2022 are currently fulfilled.
• Psychiatric & rehabilitation hospitals will be required to report once from October 1, 2022 to October 1, 2023.
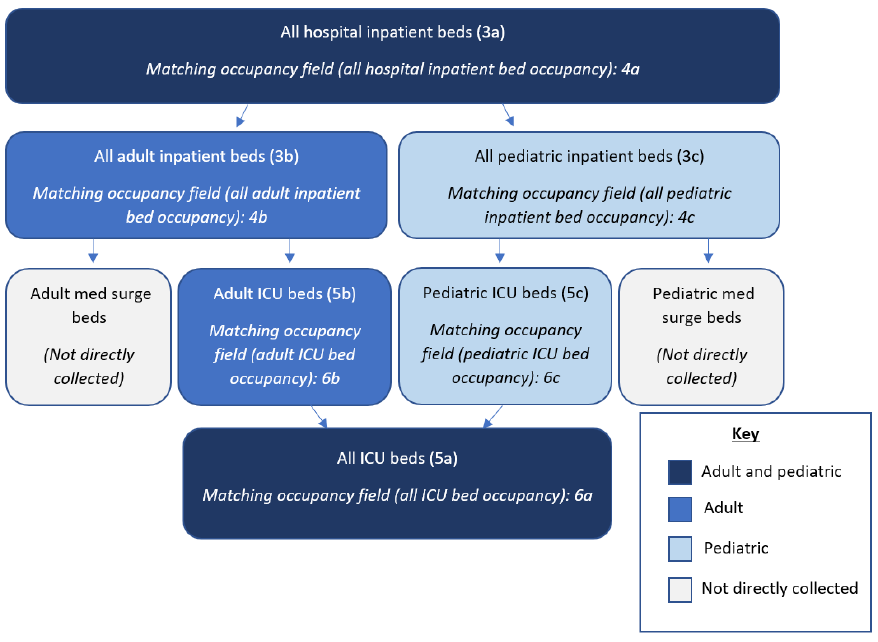
58
When psychiatric & rehabilitation hospitals report, reporting will still occur on a Wednesday, in exactly the same way as the reporting to date. The
only change federally is the reporting cadence- instead of reporting federally every week, psychiatric and rehabilitation hospitals will provide the
same snapshot once per year. The jurisdiction and/or hospital have discretion on which Wednesday during the October-October period the facility
will report on. All hospitals are asked to follow the direction of their state and jurisdiction to ensure reporting meets STLT needs.
Appendix E: Variable Relationships
Below is a simplified diagram of the relationships between variables 3a through 6c to help visually represent field subsets. Please note, we
recognize this is an oversimplification of bed types.
59
Appendix F: Template Mapping
The below table crosswalks the fields within the guidance with the data submission template. The CSV templateversion of this file is available in
the CSV Data Import section of the Hospital COVID-19 Reporting NHSN webpage: https://www.cdc.gov/nhsn/covid19/hospital-reporting.html.
Note: The template has NOT changed beyond adding new fields at the end of the template to minimize technical changes. All fields remain in the
template regardless of status.
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
reporting_for_date
N/
A
N/A
N/A
Daily
Date
hospital_name
1
a
Required
Daily
Text
ccn
1
b
Required
Daily
Text
org_id
1
c
Required
Daily
Text
state
1
d
Required
Daily
Text
county
1
e
Required
Daily
Text
zip
1
f
Required
Daily
Text
all_hospital_beds
2
a
Federally Inactive
Number
all_adult_hospital_beds
2
b
Federally Inactive
Number
all_hospital_inpatient_beds
3
a
Required
Daily
Number
all_adult_hospital_inpatient_beds
3
b
Required
Daily
Number
all_hospital_inpatient_bed_occupied
4
a
Required
Daily
Number
all_adult_hospital_inpatient_bed_occupied
4
b
Required
Daily
Number
total_staffed_icu_beds
5
a
Required
Daily
Number
total_staffed_adult_icu_beds
5
b
Required
Daily
Number
staffed_icu_bed_occupancy
6
a
Required
Daily
Number
staffed_adult_icu_bed_occupancy
6
b
Required
Daily
Number
mechanical_ventilators
7
Federally Inactive
Number
mechanical_ventilators_in_use
8
Federally Inactive
Number
total_adult_patients_hospitalized_confirmed_and_su
spected_covid
9
a
Optional
Daily
Number
total_adult_patients_hospitalized_confirmed_covid
9
b
Required
Daily
Number
total_pediatric_patients_hospitalized_confirmed_and
_suspected_covid
10
a
Optional
Daily
Number
total_pediatric_patients_hospitalized_confirmed_cov
id
10
b
Required
Daily
Number
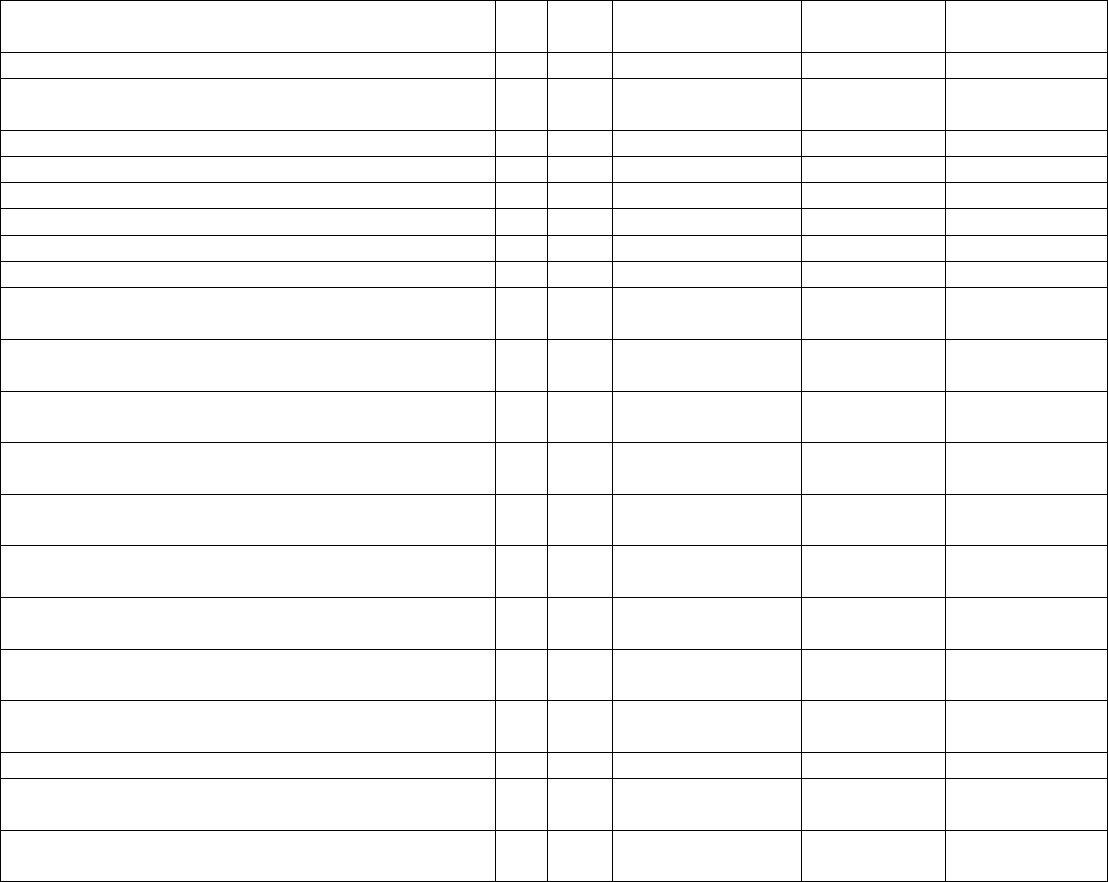
60
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
hospitalized_and_ventilated_covid_patients
11
Optional
Daily
Number
staffed_icu_adult_patients_confirmed_and_suspected
_covid
12
a
Optional
Daily
Number
staffed_icu_adult_patients_confirmed_covid
12
b
Required
Daily
Number
hospital_onset
13
Optional
Daily
Number
ed_or_overflow
14
Federally Inactive
Number
ed_or_overflow_and_ventilated
15
Federally Inactive
Number
previous_day_deaths_covid
16
Federally Inactive
Number
previous_day_admission_adult_covid_confirmed
17
a
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_18
_19
17
a-1
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_20
_29
17
a-2
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_30
_39
17
a-3
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_40
_49
17
a-4
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_50
_59
17
a-5
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_60
_69
17
a-6
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_70
_79
17
a-7
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_80
_plus
17
a-8
Required
Daily
Number
previous_day_admission_adult_covid_confirmed_un
known_age
17
a-9
Required
Daily
Number
previous_day_admission_adult_covid_suspected
17
b
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_18
_19
17
b-1
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_20
_29
17
b-2
Optional
Daily
Number
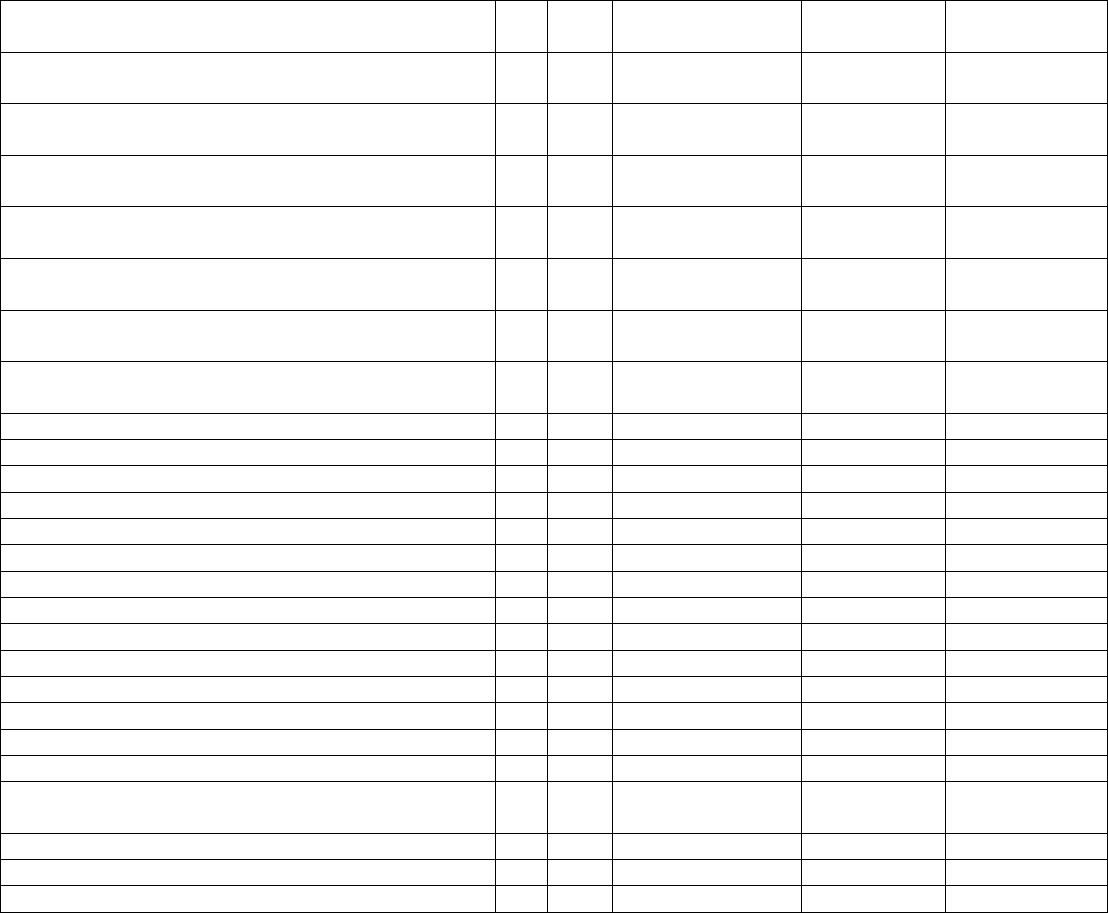
61
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
previous_day_admission_adult_covid_suspected_30
_39
17
b-3
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_40
_49
17
b-4
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_50
_59
17
b-5
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_60
_69
17
b-6
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_70
_79
17
b-7
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_80
_plus
17
b-8
Optional
Daily
Number
previous_day_admission_adult_covid_suspected_un
known_age
17
b-9
Optional
Daily
Number
previous_day_admission_pediatric_covid_confirmed
18
a
Required
Daily
Number
previous_day_admission_pediatric_covid_suspected
18
b
Optional
Daily
Number
previous_day_total_ED_visits
19
Optional
Daily
Number
previous_day_covid_ED_visits
20
Optional
Daily
Number
previous_day_remdesivir_used
21
Federally Inactive
Number
on_hand_supply_remdesivir_vials
22
Federally Inactive
Number
critical_staffing_shortage_today
23
Federally Inactive
Yes/No
critical_staffing_shortage_anticipated_within_week
24
Optional
Weekly
Yes/No
staffing_shortage_details
25
Federally Inactive
Text
PPE_supply_management_source
26
Federally Inactive
Option
on_hand_ventilator_supplies_in_days
27
a
Federally Inactive
Option
on_hand_supply_of_n95_respirators_in_days
27
b
Required
Weekly
Option
on_hand_supply_of_surgical_masks_in_days
27
c
Required
Weekly
Option
on_hand_supply_of_eye_protection_in_days
27
d
Required
Weekly
Option
on_hand_supply_of_single_use_surgical_gowns_in_
days
27
e
Required
Weekly
Option
on_hand_supply_of_gloves_in_days
27
f
Required
Weekly
Option
on_hand_supply_of_n95_respirators_in_units
28
a
Federally Inactive
Number
on_hand_supply_of_PAPR_in_units
28
b
Federally Inactive
Number
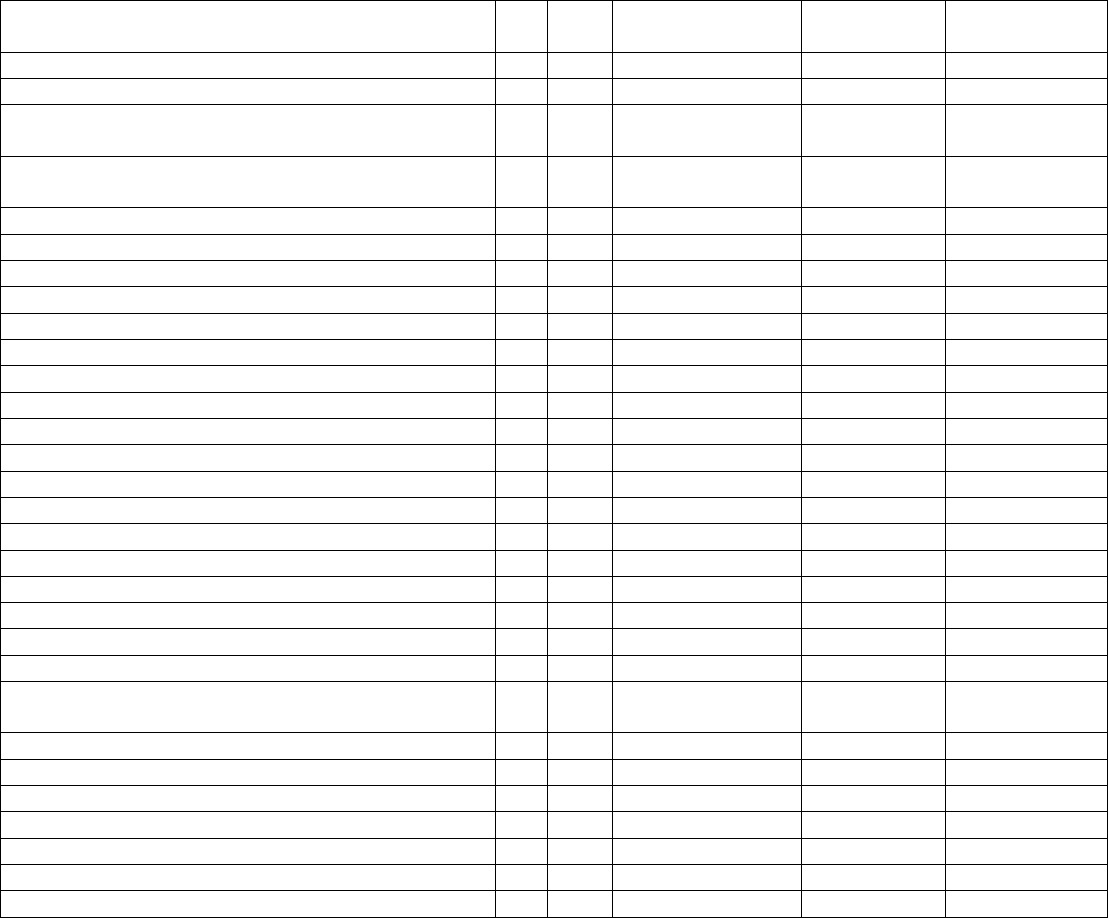
62
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
on_hand_supply_of_surgical_masks_in_units
28
c
Federally Inactive
Number
on_hand_supply_of_eye_protection_in_units
28
d
Federally Inactive
Number
on_hand_supply_of_single_use_surgical_gowns_in_
units
28
e
Federally Inactive
Number
on_hand_supply_of_launderable_surgical_gowns_in
_units
28
f
Federally Inactive
Number
on_hand_supply_of_gloves_in_units
28
g
Federally Inactive
Number
able_to_obtain_ventilator_supplies
29
a
Federally Inactive
Yes/No
able_to_obtain_ventilator_medications
29
b
Federally Inactive
Yes/No
able_to_obtain_n95_masks
29
c
Federally Inactive
Yes/No
able_to_obtain_PAPRs
29
d
Federally Inactive
Yes/No
able_to_obtain_surgical_masks
29
e
Federally Inactive
Yes/No
able_to_obtain_eye_protection
29
f
Federally Inactive
Yes/No
able_to_obtain_single_use_gowns
29
g
Federally Inactive
Yes/No
able_to_obtain_gloves
29
h
Federally Inactive
Yes/No
able_to_obtain_launderable_gowns
29
i
Federally Inactive
Yes/No
able_to_maintain_ventilator_3day_supplies
30
a
Federally Inactive
Yes/No
able_to_maintain_ventilator_3day_medications
30
b
Federally Inactive
Yes/No
able_to_maintain_n95_masks
30
c
Required
Weekly
Yes/No
able_to_maintain_3day_PAPRs
30
d
Federally Inactive
Yes/No
able_to_maintain_3day_surgical_masks
30
e
Required
Weekly
Yes/No
able_to_maintain_3day_eye_protection
30
f
Required
Weekly
Yes/No
able_to_maintain_3day_single_use_gowns
30
g
Required
Weekly
Yes/No
able_to_maintain_3day_gloves
30
h
Required
Weekly
Yes/No
able_to_maintain_3day_lab_nasal_pharyngeal_swab
s
30
i
Federally Inactive
Yes/No
able_to_maintain_lab_nasal_swabs
30
j
Federally Inactive
Yes/No
able_to_maintain_3day_lab_viral_transport_media
30
k
Federally Inactive
Yes/No
reusable_isolation_gowns_used
31
a
Federally Inactive
Yes/No
reusable_PAPRs_or_elastomerics_used
31
b
Federally Inactive
Yes/No
reusuable_n95_masks_used
31
c
Federally Inactive
Yes/No
anticipated_medical_supply_medication_shortages
32
Federally Inactive
Text
total_patients_hospitalized_confirmed_influenza
33
Required
Daily
Number
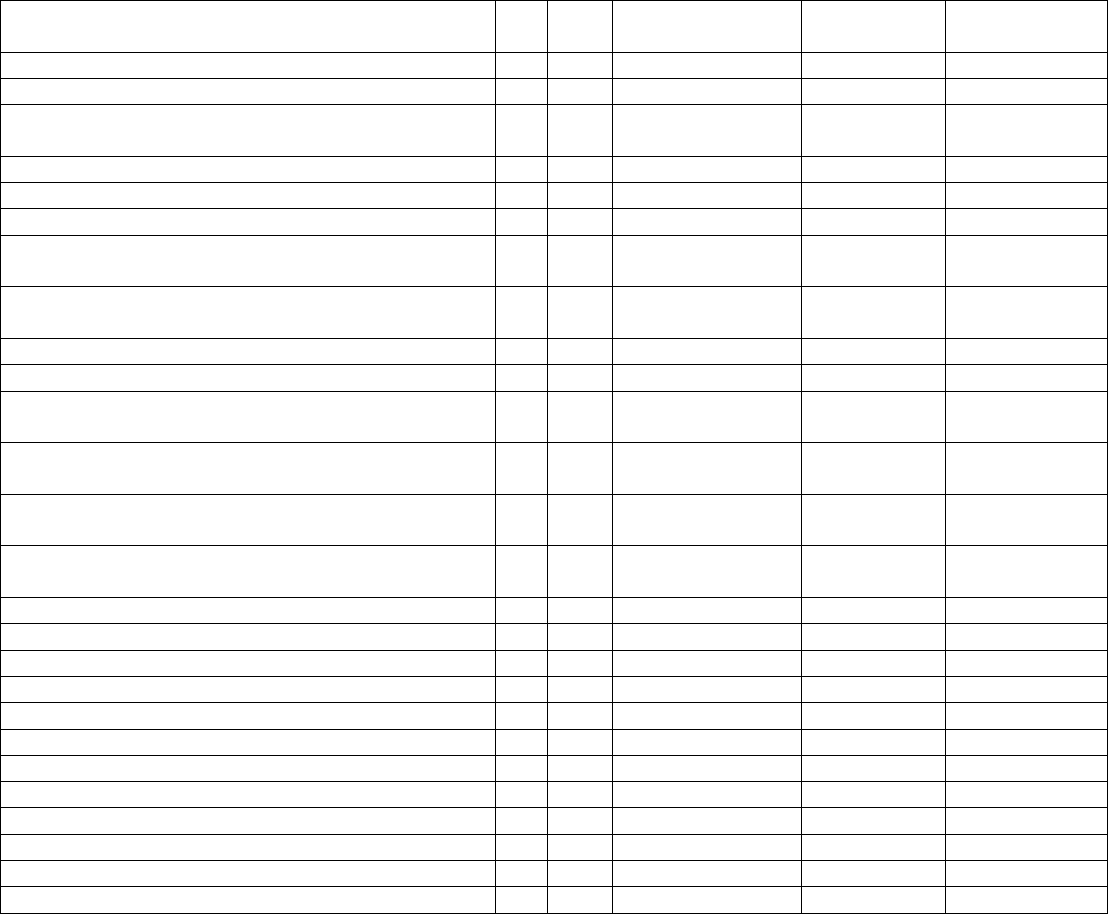
63
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
previous_day_admission_influenza_confirmed
34
Required
Daily
Number
icu_patients_confirmed_influenza
35
Required
Daily
Number
total_patients_hospitalized_confirmed_influenza_and
_covid
36
Federally Inactive
Number
previous_day_deaths_influenza
37
Federally Inactive
Number
previous_day_deaths_covid_and_influenza
38
Federally Inactive
Number
teletracking_id
1
g
Optional
Weekly
Number
on_hand_supply_Therapeutic_A_courses
39
a
Moving to HPOP
November 2, 2022
Number
previous_week_Therapeutic_A_courses_used
39
b
Moved to HPOP
November 2, 2022
Number
on_hand_supply_Therapeutic_B_courses
39
c
Federally Inactive
Number
previous_week_Therapeutic_B_courses_used
39
d
Federally Inactive
Number
on_hand_supply_Therapeutic_C_courses
40
a
Moved to HPOP
November 2, 2022
Number
previous_week_Therapeutic_C_courses_used
40
b
Moved to HPOP
November 2, 2022
Number
on_hand_supply_Therapeutic_D_courses
40
c
Moved to HPOP
November 2, 2022
Number
previous_week_Therapeutic_D_courses_used
40
d
Moved to HPOP
November 2, 2022
Number
on_hand_supply_Therapeutic_E_courses
40
e
Federally Inactive
Number
previous_week_Therapeutic_E_courses_used
40
f
Federally Inactive
Number
on_hand_supply_Therapeutic_F_courses
40
g
Federally Inactive
Number
previous_week_Therapeutic_F_courses_used
40
h
Federally Inactive
Number
on_hand_supply_Therapeutic_G_courses
40
i
Federally Inactive
Number
previous_week_Therapeutic_G_courses_used
40
j
Federally Inactive
Number
on_hand_supply_Therapeutic_H_courses
40
k
Federally Inactive
Number
previous_week_Therapeutic_H_courses_used
40
l
Federally Inactive
Number
on_hand_supply_Therapeutic_I_courses
40
m
Federally Inactive
Number
previous_week_Therapeutic_I_courses_used
40
n
Federally Inactive
Number
on_hand_supply_Therapeutic_J_courses
40
o
Federally Inactive
Number
previous_week_Therapeutic_J_courses_used
40
p
Federally Inactive
Number
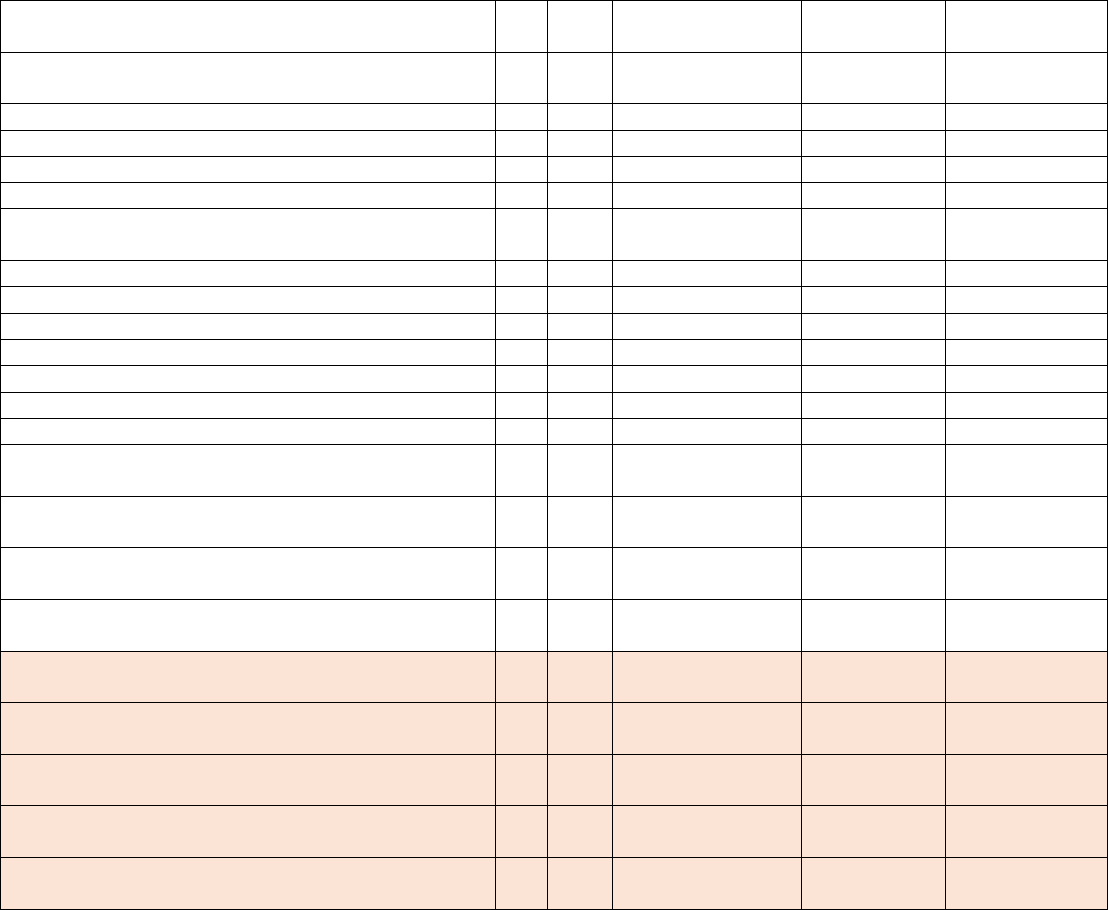
64
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
previous_week_personnel_covid_vaccinated_doses_
administered
41
Federally Inactive
Number
total_personnel_covid_vaccinated_doses_none
42
Federally Inactive
Number
total_personnel_covid_vaccinated_doses_one
43
Federally Inactive
Number
total_personnel_covid_vaccinated_doses_all
44
Federally Inactive
Number
total_personnel
45
Federally Inactive
Number
previous_week_patients_covid_vaccinated_doses_on
e
46
Federally Inactive
Number
previous_week_patients_covid_vaccinated_doses_all
47
Federally Inactive
Number
hhs_id
1
h
Optional
Daily
Text
all_pediatric_inpatient_beds
3
c
Required
Daily
Number
all_pediatric_inpatient_bed_occupied
4
c
Required
Daily
Number
total_staffed_pediatric_icu_beds
5
c
Required
Daily
Number
staffed_pediatric_icu_bed_occupancy
6
c
Required
Daily
Number
staffed_icu_pediatric_patients_confirmed_covid
12
c
Required
Daily
Number
previous_day_admission_pediatric_covid_confirmed
_0_4
18
c-1
Required
Daily
Number
previous_day_admission_pediatric_covid_confirmed
_5_11
18
c-2
Required
Daily
Number
previous_day_admission_pediatric_covid_confirmed
_12_17
18
c-3
Required
Daily
Number
previous_day_admission_pediatric_covid_confirmed
_unknown
18
c-4
Required
Daily
Number
[NEW]
numConfFluHospPatInPtBedAdult
33
a
Optional
Daily
Number
[NEW]
numConfFluHospPatInPtBedPed
33
b
Optional
Daily
Number
[NEW]
numConfFluNewAdmInPtBedAdult
34
a
Optional
Daily
Number
[NEW]
numConfFluNewAdmInPtBedPed
34
b
Optional
Daily
Number
[NEW]
numConfFluPatICUBedAdult
35
a
Optional
Daily
Number
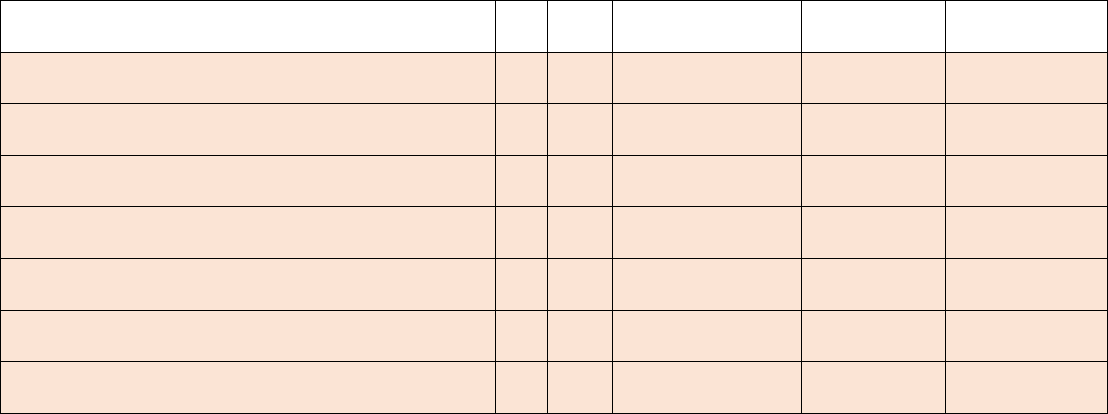
65
Template Data Element Name
ID
Sub
ID
Status
Cadence
Format
[NEW]
numConfFluPatICUBedPed
35
b
Optional
Daily
Number
[NEW]
numConfRSVHospPatsAdult
48
a
Optional
Daily
Number
[NEW]
numConfRSVHospPatsPed
48
b
Optional
Daily
Number
[NEW]
numConfRSVNewAdmAdult
49
a
Optional
Daily
Number
[NEW]
numConfRSVNewAdmPed
49
b
Optional
Daily
Number
[NEW]
numConfRSVICUPatsAdult
50
a
Optional
Daily
Number
[NEW]
numConfRSVICUPatsPed
50
b
Optional
Daily
Number
